Bonding and Structure Covalent Bonds
advertisement
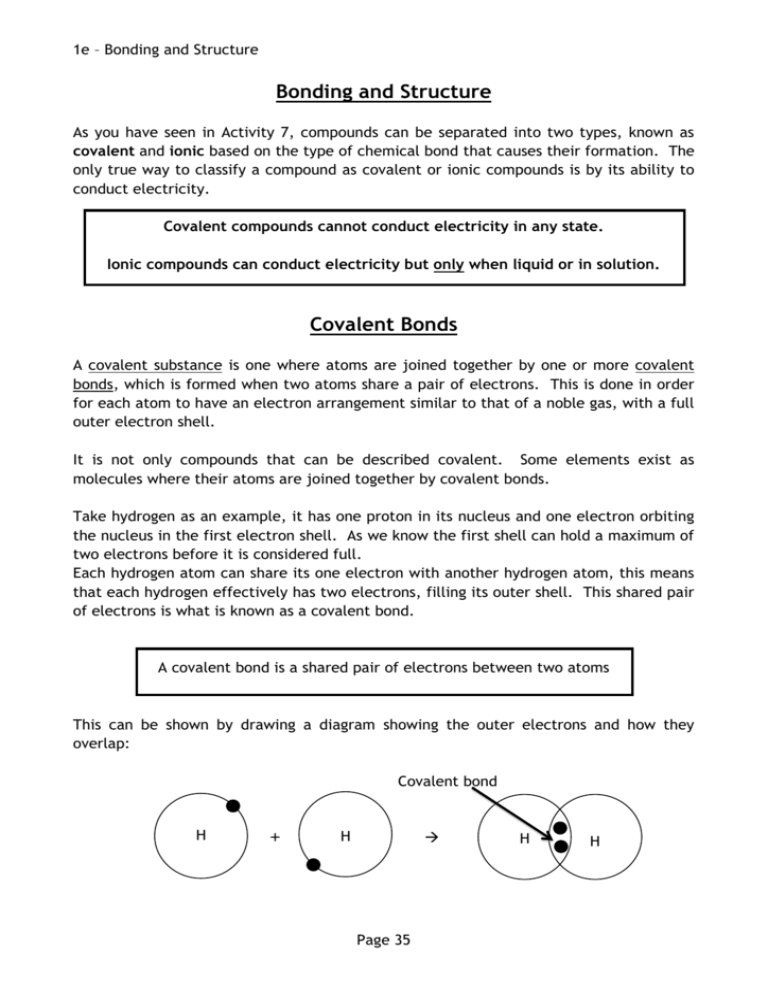
1e – Bonding and Structure Bonding and Structure As you have seen in Activity 7, compounds can be separated into two types, known as covalent and ionic based on the type of chemical bond that causes their formation. The only true way to classify a compound as covalent or ionic compounds is by its ability to conduct electricity. Covalent compounds cannot conduct electricity in any state. Ionic compounds can conduct electricity but only when liquid or in solution. Covalent Bonds A covalent substance is one where atoms are joined together by one or more covalent bonds, which is formed when two atoms share a pair of electrons. This is done in order for each atom to have an electron arrangement similar to that of a noble gas, with a full outer electron shell. It is not only compounds that can be described covalent. Some elements exist as molecules where their atoms are joined together by covalent bonds. Take hydrogen as an example, it has one proton in its nucleus and one electron orbiting the nucleus in the first electron shell. As we know the first shell can hold a maximum of two electrons before it is considered full. Each hydrogen atom can share its one electron with another hydrogen atom, this means that each hydrogen effectively has two electrons, filling its outer shell. This shared pair of electrons is what is known as a covalent bond. A covalent bond is a shared pair of electrons between two atoms This can be shown by drawing a diagram showing the outer electrons and how they overlap: Covalent bond H + H Page 35 H H 1e – Bonding and Structure There are also examples of substances where two or more covalent bonds form between the same two atoms, these are known as double and triple covalent bonds. For example oxygen and nitrogen both exist as diatomic molecules, nitrogen has five outer electrons and oxygen has six outer electrons. When an oxygen atom forms a diatomic molecule it must share two of its electrons with another oxygen atom, this forms a double covalent bond. Double covalent bond + O O O O When a nitrogen atom forms a diatomic molecule it must share three of its electrons with another oxygen atom, this forms a triple covalent bond. Complete this diagram by adding the electrons to show a triple covalent bonded nitrogen molecule: N + N N Draw another diagram for the formation of hydrogen chloride: Page 36 N 1e – Bonding and Structure Valency for Covalent Substances When covalent compounds are formed, atoms share electrons with one another in order to achieve a full outer shell. Every electron shared becomes part of a pair (a covalent bond), which allows atoms to gain the share of electrons from other atoms until a full outer shell is achieved. Since covalent compounds normally form between non-metal elements we will examine groups 4 to 0. Complete this table: Group 4 5 6 7 0 Example element C N O F Ne Outer electrons 5 Electrons needed to complete outer shell 3 Valency Number 3 Diatomic Elements When two atoms join together they form a molecule. A molecule can be of an element, where all atoms are the same, or a compound, where the atoms are different. There are seven elements that, under normal conditions, exist as “diatomic molecules”. This means that two of their atoms join together to form a molecule When these seven are present as an element their formula is written to reflect the fact that they exist as diatomic molecules. You must learn these seven elements, they are: Hydrogen, H2 Chlorine, Cl2 Oxygen, O2 Bromine, Br2 Nitrogen, N2 Iodine, I2 Fluorine, F2 Page 37 1e – Bonding and Structure Attraction and Repulsion When any two charged particles are close together they will interact with one another, either attracting or repelling each other. If both particles are positive or both are negative then they will repel one another. e.g. two protons will repel one another. If the particles have opposite charges then they will attract one another. e.g. a proton and an electron will be attracted to one another. Like charges repel and opposite charges attract So when two atoms come together, how can the covalent bond hold the two atoms together? Why don’t the two positive nuclei repel one another and fly apart? This is because a balance is reached between attraction and repulsion. The two positive nuclei will repel one another but they both have a shared attraction for the pair of bonding electrons. As a result the two atoms are held in a stable formation. Complete the diagram, showing the repulsion of the two nuclei and their shared attraction for the bonding electrons: + + Page 38 1e – Bonding and Structure Covalent Structures As we have seen, some compounds exist as covalent molecular compounds. These molecules have very weak forces of attraction between the molecules but strong covalent bonds inside the molecules. When melting or boiling a covalent molecular substance it is only the weak forces of attraction that must be broken, not the strong covalent bonds. As a result covalent molecular compounds have low melting and boiling points and can exist as solids, liquids and gases at room temperature. Some covalent substances exist as giant three-dimensional covalent networks. This is where the atoms are joined together by covalent bonds but rather than being in molecules the atoms bond with one another in a continual way. Carbon can exist in many different forms, one of which is diamond. Diamond is a three-dimensional network of carbon atoms where each carbon has four covalent bonds joining it to 4 different carbons. When melting or boiling a covalent network substance it is only the strong covalent bonds that must be broken. As a result covalent network compounds have very high melting and boiling points and can only exist as solids at room temperature. Page 39 1e – Bonding and Structure Molecule v Network Physical Properties Carbon and silicon are both found in group four of the periodic table and both form covalent compounds with oxygen. Let’s compare carbon dioxide with silicon dioxide: Carbon dioxide Silicon dioxide Exists as covalent molecules Exists as a covalent network Gas at room temperature Solid at room temperature Melting Point: -78 °C Melting point: 1700 °C Boiling Point: -57 °C Boiling point: 2230 °C Chemical Formulae For covalent molecular substances the chemical formula tells us the number of atoms of each element present in the molecule e.g. carbon dioxide is CO2 this tells us that there is one carbon atom and two oxygen atoms in the molecule. Water is H2O and this tells us that there are two hydrogen atoms and one oxygen atom in the molecule. For covalent network substances the formula tells us the ratio of the atoms in the network, not the definite number present. e.g. silicon dioxide is SiO2, this tells us that there is one silicon atom for every two oxygen atoms in the network. Page 40 1e – Bonding and Structure Summary – Covalent Substances covalent network (x3) conduct pair solid molecular (x3) electrons (x2) elements attraction ratio Covalent substances can be either ____________________ or compounds. They are formed when atoms form ____________________ bonds. A covalent bond is described as a shared ____________________ of ____________________ between two atoms. A covalent bond holds the atoms together due to the two nuclei having a shared ____________________ for the pair of ____________________. Covalent compounds can be easily distinguished from ionic compounds, as they cannot ____________________ electricity in any state. A covalent substance can form one of two structures, it can be either a covalent ____________________ or ____________________. These two structures are distinguished by their physical properties; covalent ____________________ have low melting and boiling points and can exists as solids, liquids or gases at room temperature, while covalent ____________________ have very high melting and boiling points and only exist as ____________________ at room temperature. The formula of a covalent ____________________ substance gives the exact number of atoms present inside the molecule, whilst a covalent ____________________ structure gives the ____________________ of atoms in the network. Page 41 1e – Bonding and Structure Ionic Bonding An ionic substance is one that is made up, not of atoms, but of ions. As covered previously an ion is a charged particle, formed when an atom either gains or loses electrons. Similarly to covalent bonding, atoms need to achieve a full outer electron shell, similar that of their nearest noble gas. Unlike covalent bonding, however, no electrons are shared to form a bond. Instead one atom will donate electrons to the other forming a positive ion (having lost electrons) and a negative ion (having gained electrons). An ionic bond is simply an electrostatic attraction between a positive and a negative ion. Take sodium chloride as an example. Sodium has an electron arrangement of 2,8,1 and chlorine has an arrangement of 2,7. Sodium needs to lose one electron to achieve an arrangement of 2,8 and chlorine needs to gain one electron to also achieve an arrangement of 2,8. 1e e.g. + + Empty shell Cl Na + + attraction Full outer shell Na+ Cl- Another key difference from covalent bonding is that ionic bonding can only occur in compounds, not in elements. Page 42 1e – Bonding and Structure Ionic Structure The structure adopted by ionic compounds is known as a crystal lattice structure. Each ion is surrounded by the ion of opposite charge forming a very rigid and well-ordered structure. Different compounds will form lattices of different shapes and it is this arrangement of ions that causes ionic compounds to form crystals of various shapes. Sodium chloride forms a cubic shaped crystal lattice where every sodium ion and every chloride ion is surrounded on six sides by an oppositely charged ion When melting or boiling an ionic substance it is the strong ionic bonds that must be broken. As a result ionic compounds have high melting and boiling points and only exist as solids at room temperature. This is an image of a sodium chloride crystal. You can see that it forms a cubic shape, just like the lattice shape above. Although ionic compounds have high melting points they are lower that that of a covalent network. Below is a comparison of sodium chloride and silicon dioxide. Structure Melting point / °C Boiling point / °C Sodium chloride crystal lattice 801 1413 Silicon dioxide covalent network 1700 2230 Many ionic compounds will dissolve in water, they are said to be soluble. The reason for their solubility is that, when added to water, the crystal lattice structure breaks down and the ions are free to move in the solution. Page 43 1e – Bonding and Structure Valency and Ionic Compounds For ionic compound the valency number is the number of electrons that must be lost or gained in order to form a full outer shell. Group 4 and 0 atoms do not form ions. Group Example element Outer electrons 1 2 3 Na Mg 1 2 4 5 6 7 Al P S Cl 3 5 6 7 Electrons gained or lost 2 lost Charge 2+ – Valency 2 1 No Ions Page 44 1 gained 0 No Ions 1e – Bonding and Structure Summary – Ionic Compounds Ionic positive oppositely soluble attraction crystal high negative atoms ion electrons substances are made ____________________ up forms of when ions not ____________________. an atom either gains or An loses ____________________. During a chemical reaction this involves one atom donating its electrons, forming a ____________________ ion, to another atom, forming a ____________________ ion. An ionic bond is the ____________________ that the positive and negative ions have for one another. The structure of an ionic compound is known as a ____________________ lattice structure and consists of a well-ordered, three-dimensional lattice of ____________________ charged ions. When an ionic substance is melted or boiled, strong ionic bonds must be broken and, as a result, these substances have ____________________ melting and boiling points. A liquid (or molten) ionic compound has lost its rigid and well-ordered structure and the ions are able to move over one another and flow. Another way to break down the lattice structure is to dissolve the substance. If the substance is ____________________ in water then the lattice structure is lost and the ions are again able to flow in the solution. Page 45 1e – Bonding and Structure Electricity and Structure An electrical current is defined as a flow of charged particles For any substance to be an electrical conductor it must have charged particles, which are free to move. Metals and carbon in the form of graphite have electrons, which are free to move, and this is why metals and graphite are conductors of electricity. Ionic compounds, as we have seen, are made of charged particles known as ions. Ionic compounds can only conduct electricity when they are molten or in solution. Why is this? Ionic substances have a rigid crystal lattice structure, however when they are melted or dissolved this rigid structure is lost. This image shows sodium chloride dissolving. When added to water the ions are surrounded by water molecules and are able to leave the lattice structure. Ionic compounds can conduct electricity only as a liquid or when in solution because they have ions that are free to move. Solid ionic compounds contain ions, but these ions are rigidly held in place and are unable to move Covalent compounds, both molecular and network are made of atoms, which we learned earlier must have a neutral charge, and not ions. Without charged particles it is not possible for covalent compounds to conduct electricity. Covalent compounds cannot conduct electricity, as they contain no ions. Check Test 1.12 Page 46 1f – Formulae and Reaction Quantities Ionic Formulae When working with an ionic compound it is important to be able to understand and write an ionic formula. An ionic formula gives both the charge on the ions in the compound and the ratio of the ions in the crystal lattice. Writing an ionic formula links together two skills you already have: 1. To write a chemical formula using valency rules, group ions and Roman numerals where appropriate 2. To work out the charge of an ion using the periodic table and electron arrangements and write the symbol for the ion. e.g. sodium chloride 1. Write the formula 1 1 Na Cl Na1 Cl 1 Na Cl 2. Write the symbols for the ions Na – 2,8,1 – loses 1 e – Na+ Cl – 2,8,7 – gains 1 e – ClTo write the ionic formula you simply write the ions in the ratio of the chemical formula: Na Cl is in the ration 1:1 which gives an ionic formula of Na+ ClIf any ion is in the formula with a number other than one it must be put into brackets. e.g. Magnesium bromide is MgBr2 the ions are Mg2+ and Br- so the ionic formula is Mg2+(Br-)2 Give the ionic formula of the following compounds: a) magnesium nitrate b) sodium oxide c) aluminium oxide d) iron (II) sulphide Check Test 1.13 Page 47 1f – Formulae and Reaction Quantities Chemical Equation Chemical reactions are often described in the form of a chemical equation. A chemical equation is just like a word equation, except written with chemical formulae. When writing a chemical formula it is important to first write a word equation, then simply replace each chemical name with its chemical formula. Note that if any of the seven diatomic elements are present as elements, their formula reflects the fact that they are diatomic. e.g. hydrogen is written as H2. For example: magnesium + Mg soidum hydroxide NaOH + bromine magnesium bromide Br2 MgBr2 + hydrochloric acid + HCl sodium chloride NaCl + water + H 2O For each of the following word equations, write the formula equation: a) zinc + copper chloride zinc(II) chloride + copper b) lithium sulphate + barium chloride lithium chloride + barium sulphate c) carbon + oxygen carbon dioxide d) iron (II) oxide + carbon monoxide iron + carbon dioxide e) hydrogen + chlorine hydrogen chloride Page 48 1f – Formulae and Reaction Quantities Showing the State When writing chemical formulae it can be useful to also show the state of matter in which the chemical would be. This is done using state symbols after the chemical formula. State of Matter State symbol solid (s) liquid/molten (l) gas (g) in solution (aqueous) (aq) For example if solid sodium reacts with water to form hydrogen gas and a solution of sodium hydroxide, we could describe this reaction as: Na(s) + H2O(l) NaOH(aq) + H2(g) 1. Complete the following examples to show their state symbol. a) solid sulphur b) liquid nitrogen c) ice d) magnesium chloride solution 2. Write a formula equation including state symbols. a) Copper(II) chloride solution reacts with sodium carbonate solution to form sodium chloride solution and solid copper(II) carbonate. b) Copper(II) carbonate powder reacts with hydrochloric acid solution (HCl) to form copper(II) chloride solution, water and bubbles of carbon dioxide. Check Test 1.14 Page 49 1f – Formulae and Reaction Quantities Finding a Balance As you have seen, a chemical reaction can be described through the use of both word equations and formula equations. These two methods rely on the same idea, reactants go into the reaction and products come out. When you look at some formula equations you might notice that not everything is balanced. For example let’s look at the reaction of hydrogen and oxygen to make water: hydrogen + oxygen + H2 O2 water H2O As you can see diatomic molecules of hydrogen and oxygen react together to form a water molecule. We can show this as a diagram: = an atom of hydrogen = an atom of oxygen H2 + O2 H2O There is an imbalance between the reactants and the products, as there are two hydrogen atoms and two oxygen atoms on the reactants side, but only one oxygen atom and two hydrogen atoms on the products side. This means that the equation needs to be balanced. In order to balance an equation you must follow some rules: 1. First count the number of atoms of each element on both sides of the equations, this can be noted down in a small table. H2 + H 2 O2 H2O H 2 O 2 Page 50 O 1 1f – Formulae and Reaction Quantities 2. By looking at your count, decide which elements are lacking and whether they are lacking as reactants or products. There is a shortage of oxygen on the products side. 3. Next you can add reactants or products to the equation in order to correct the imbalance. Whenever you add something you must recount including the atoms you have added. Note that only complete ‘formula units’ can be added, you cannot just add an atom of an element unless it is a reactant or product of the reaction. H2 + H 2 O2 H2O +H2O H 2 4 O 2 O 1 2 4. Now you can repeat step 2 and 3 until the count is equal on both sides. There is a shortage of hydrogen on the reactants side. H2 +H2 + H 2 4 O2 H2O +H2O H 2 4 O 2 2 O 1 2 5. The count is now balanced, with four hydrogen atoms and two oxygen atoms on either side. You can now rewrite the equation totalling up the number of each reactant and product, as with formulae the number 1 need not be written. 2H2 + O2 This is a balanced equation. Page 51 2H2O 1f – Formulae and Reaction Quantities Balance the following equations: 1. H2 + Cl2 HCl 2. CH4 + O2 CO2 3. CO + O2 CO2 4. Al(OH)3 + HCl 5. Fe O2 Fe2O3 + + H2O AlCl3 + Check Test 1.15 Page 52 H2O 1f – Formulae and Reaction Quantities Gram Formula Mass Earlier in this topic you saw that every atom of an element has mass, due to protons and neutrons in the nucleus, and that an average of all possible isotopes is called the Relative Atomic Mass or R.A.M. Using these masses and the chemical formula it is possible to calculate the Gram Formula Mass or GFM. Due to the incredibly small size of atoms it is not logical to try and measure the mass of one atom or even a few million atoms. Instead we measure the mass of one mole of atoms. The term mole simply represents a number, an extremely large number and by measuring this quantity of atoms and molecules we can measure sensible and easy to handle masses. The GFM is defined as the mass of one mole of a substance. To calculate the GFM you must start with the correct chemical formula. 1. First, write down the chemical formula of the relevant substance e.g. H2O 2. Using the data booklet, note the RAM of each element in the substance e.g. H = 1 O = 16 3. Add up the total mass of the substance taking into account the chemical formula e.g. H2O : 2xH=2x1=2 1 x O = 1 x 16 = 16 Total = 18 The GFM of water is 18 which means that one mole of water has a mass of 18g. Note that if the formula has brackets then the number out of the brackets multiplies everything inside of the brackets. e.g. Mg(NO3)2 is 1 x Mg, 2 x N and 6 x O. Calculate the GFM of the following substances: a) NaCl b) O2 c) AgCl d) Al2(SO4)3 Check Test 1.16 Page 53 1f – Formulae and Reaction Quantities Mass and Moles Knowing the formula of a substance, you can now calculate its GFM. This forms a relationship with the mass of a substance (m) and the number of moles (n) of a substance as shown on page 1 of the data booklet n m GFM number of moles = mass of a substance / GFM This can be rearranged to give m n x GFM e.g. If you were asked to weigh out 2 moles of sodium chloride, what mass would you collect? m e.g. mass of a substance = number of moles / GFM = n x GFM = 2 x 58.5 = 117 g GFM = NaCl = 23 + 35.5 = 58.5 If you measured out 50 g of calcium carbonate, how many moles of calcium carbonate have you collected? n = m / GFM = 50 / 100 = 0.5 mol GFM = CaCO3 = 40 + 12 + (3 x 16) = 100 Calculate the mass of: a) 0.1 mol of O2 b) 5 mol of NaBr c) 3.5 mol of CuCl2 d) 0.5 mol of K2SO4 Page 54 1f – Formulae and Reaction Quantities Calculate the number of moles in: a) 10 g of H2 b) 12.8 g of SO2 c) 20 g of He d) 12.7 g of I2 Check Test 1.17 Page 55 1f – Formulae and Reaction Quantities Concentration Concentration (C) is a measure of the quantity of chemical present in a given volume. Chemical concentration is normally given as the number of moles in a litre of a solution, mol l-1 (moles per litre). This forms a relationship with the volume of a solution (V) and the number of moles (n) of a substance as shown on page one of the data booklet nCV number of moles = concentration x volume This can be rearranged to give C n V concentration = number of moles / volume n C volume = number of moles / number of moles and V The volume must be in litres before being used in the calculation. To change a volume in cm3 into litres we must divide by 1000. e.g 100 cm3 = 100 ÷ 1000 = 0.1 litres Convert the following volumes into litres: a) 75 cm3 = b) 150 cm3 = c) 10 cm3 = d) 700 cm3 = Page 56 1f – Formulae and Reaction Quantities e.g. If you were asked to make a 500 cm3 solution containing 2 moles of sodium chloride, what concentration would this solution have? c e.g. 500 cm3 =n/v = 2 / 0.5 = 4 mol l-1 = 500 / 1000 = 0.5 litres If you had 750cm3 of a 1.5 mol l-1 solution, how many moles of the chemical would you have? n 750 cm3 =cxv = 0.75 x 1.5 = 1.125 mol = 750 / 1000 = 0.75 litres Calculate the concentration of a 800 cm3 solution containing: a) 1.5 moles of MgCl2 b) 5 mol of NaBr c) 3.5 mol of CuCl2 d) 0.5 mol of K2SO4 Calculate the number of moles in a 200 cm3 solution with a concentration of: e) 2 mol l-1 f) 1.4 mol l-1 g) 0.01 mol l-1 h) 0.05 mol l-1 Page 57 1f – Formulae and Reaction Quantities Concentration and Mass If we look at both of our calculations side by side we can see that they have one variable in common, the number of moles (n). n m GFM nCV With this common link, we can now know more about a solution by using both calculations together. e.g. e.g. If you were asked to make a 500 cm3 solution of sodium chloride with a concentration of 1.4 mol l-1, what mass of sodium chloride would you need? n =cxv = 1.4 x 0.5 = 0.7 mol 500 cm3 = 500 / 1000 = 0.5 litres m = n x GFM = 0.7 x 58.5 = 40.95 g GFM = NaCl = 23 + 35.5 = 58.5 If you were to make 750cm3 of a potassium sulphate (K2SO4) solution using 17.4 g of solid potassium sulphate, what concentration would the solution have? n = m / GFM = 17.4 / 174 = 0.1 mol GFM = K2SO4 = 78 + 32 +64 = 174 c =n/v = 0.1 x 0.75 = 0.075 mol l-1 750 cm3 = 750 / 1000 = 0.75 litres Page 58 1f – Formulae and Reaction Quantities Calculate the mass of magnesium chloride (MgCl2) required to make a 1.5 litre solution with a concentration of: a) 2 mol l-1 b) 1.4 mol l-1 c) 0.01 mol l-1 d) 0.05 mol l-1 Calculate concentration of a 350 cm3 solution containing: a) 20.6 g of sodium bromide (NaBr) b) 4 g of ammonium nitrate (NH4NO3) c) 6.38 g of copper(II) sulphate d) 21.2 g of sodium carbonate Check Test 1.18 Page 59