Name Hour________ Gases Homework Packet Intro 1. What are the
advertisement

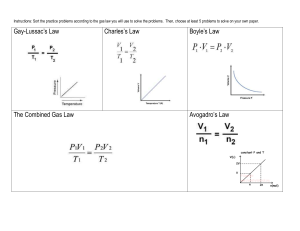
Name __________________________________ Hour________ Gases Homework Packet Intro 1. What are the 4 physical properties of gases? 2. Describe why Boyle’s Law is true using the kinetic molecular theory 3. Describe why Charles’ Law is true using the kinetic molecular theory 4. Describe why Gay-Lussac’s Law is true using the kinetic molecular theory 5. Draw a graph you would expect to see for each of the three laws and state the relationship between the variables (inverse or direct) Boyles Charles Gay-Lussacs 6. What does STP stand for? What are the values for STP? Boyles Law 7. A 412 mL balloon at atmospheric pressure (1 atm) is placed in a vacuum jar and the air is evacuated from the jar. If the balloon enlarges to a volume of 663 mL, then what is the new pressure? 8. A book is placed on top of a syringe in a chemistry lab. The volume of air in the syringe is 25.1 mL when the pressure is 98 kPa. What volume would the air occupy when the pressure is increased to 130 kPa. 9. A sample of nitrogen gas originally has a volume of 416 mL at a pressure of 827 mmHg. What is the volume if the pressure changes to 682 mmHg? 10. A balloon contains 3.55 L of neon gas at 24°C and 1.5 atm. What would be the volume of the gas if its pressure changes to 0.80 atm but the temperature remains the same? Charles’ Law 11. 515 K = ________°C _______K = -30°C 273K = ________°C 12. A hot air balloon has a volume of 3125 L at 40°C. What temperature would be required to give it a volume of 3952 L? 13. You need 6.22 L of SO2 gas at 0°C and 1 atm. What would be the volume of this gas at 25°C and 1 atm? 14. A rubber balloon occupies 400 mL when held at a temperature of 25°C. What volume will it occupy when placed in a liquid nitrogen bath at a temperature of -100°C? 15. A balloon with a volume of 2.0L and a temperature of 25°C is submerged in a tank of liquid nitrogen that is at a temperature of 80K. What will be its new volume? Gay-Lussac’s Law 16. A whip cream aerosol can ahs a volume of 350 mL and a pressure of 2.50 atm in the refrigerator at 5°C. What pressure would be exerted on this can if it is thrown into a fire and heated to 650°C? 17. Suppose a tennis ball contains 119 mL of gas and is pressurized to 220 kPa at a temperature of 41°C. What is the internal pressure of the ball if the temperature drops to 3°C? 18. The metal lid on a plastic tennis ball container is sealed at 2.3 atm of pressure at a temperature of 25°C. The container is placed in the trunk of a car on a cold, frigid day (T=-2°C). What is the pressure of gas inside the can at this temperature? Misc Gas Law Problems 19. A balloon with a volume of 300mL at 30°C expands to 1.0L. At what temperature did this occur? 20. If 6.22 L of Cl2 gas is at a pressure of 642 mmHg, then what must the pressure become to change the volume to 7.64 L? 21. The gas in a balloon has a volume of 4.0L at 120 kPa. The balloon is released into the atmosphere and experiences 105 kPa. What is the new volume of the balloon? 22. What would be the original Celsius temperature of a gas at a pressure of 1.75 atm if the temperature changes to 56°C and the pressure to 2.34 atm? Combined Gas Law 23. A gas has a volume of 18.5 L at 85.5 kPa and 296K. What is the new volume of the gas at STP? 24. A balloon full of He gas has a volume of 132 L at 99.7 kPa and 30°C. What temperature is required for the balloon to have a volume of 176 L at a pressure of 77.6 kPa? 25. A bag is inflated to a volume of 3.89 L at 111 kPa and 23°C. If the volume drops to 3.05 L at a temperature of 4°C, then what is the new pressure? 26. A bicycle container has 2.3 L of air at 28°C and 5.2 atm. What volume will the air sample occupy at 5°C and 4.8 atm? Ideal Gas Law 27. 1 mole of ANY gas @ STP = ______________ L 28. How many moles of nitrogen gas are in a balloon that has a volume of 15.9 L at a pressure of 149 kPa and a temperature of 28°C? 29. The red carbon dioxide fire extinguisher on the wall has a volume of 5.25 L. Assuming the gas is at 23°C and 8 atm, how many moles of CO2 are in the fire extinguisher? How many grams? 30. There are 2.20 mol of NH3 gas in a tank that has a pressure of 199 kPa and a temperature of 25°C. What is the volume of the tank? Daltons/Grahams Law 31. Rank the following as to which gas would effuse the fastest (1-fastest, 5-slowest) ____N2 ____CO2 ____SO2 ____CH4 ____Cl2 32. The total pressure inside of a tank containing oxygen, nitrogen, and hydrogen is 730 mmHg. The oxygen has a partial pressure of 220 mmHg and the nitrogen has a partial pressure of 450 mmHg. What is the pressure of the hydrogen gas in the tank? Gas Stoichiometry 33. How many liters of hydrogen gas will be produced at 0.987 atm and 305 K if 30 g of HCl reacts with excess Na according to the following equation: 2Na + 2HCl 2NaCl + H2 34. A rocket contains 3000 grams of ethane (C2H6) and sufficient liquid oxygen for complete combustion. The reaction taking place in the rocket engine is 2C2H6 + 7O2 4CO2 + 6H2O What volume (in liters) at STP of carbon dioxide are produced by the reaction of 3000 grams of ethane?