Gases II Notes - Oregon State University
advertisement

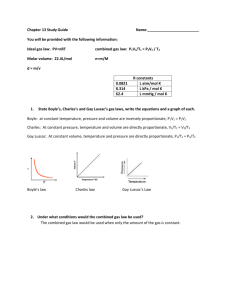
Stoichiometry Problems Volume of CO2 (g) produced from the combustion of one gallon of gasoline: CH 222 Octane to CO2 Notes Oregon State University Dr. Richard Nafshun A couple of CH 222 students hop into a Toyota and drive to Eugene to visit an unenlightened friend. Using a few opportune reaction conditions, determine the liters of CO2 produced. What is the length (in feet) of a cubic container that would hold the CO2? Molar Mass of Octane = 114.2285 g/mol 1.00000 Gallons = 3.78541 Liters 1.0000000 Liter = 0.03531467 cubic foot Density of octane = 0.703 g/ml Mileage Est. (mpg city/highway): 32/41 1 a. I will assume the combustion of 1.00 gallon of octane and the existence of CO2 (g) at 298 and 1 atm when at equilibrium: 3.785 L = 3.785 L C8H18 1.00 gal C8H18 1.000 gal b. 1000 mL = 3785 mL C8H18 3.785 L C8H18 1L c. 0.703 g = 2660 g C8H18 3785 mL C8H18 1 mL 1. 1 mol = 23.3 mol C8H18 2660 g C8H18 114.21 g 2. 8 mol CO 2 = 186 mol CO2 23.3 mol C8H18 1 mol C 8 H18 d. PV = nRT L atm )(298 K ) (186mol)(0.0821 nRT mol K = 4550 L CO2 (g) V= = (1.00 atm) P 2 [Change L to ft3 and discuss a cube that holds the carbon dioxide]: 0.0353 cubic feet = 161 ft3 CO2 (g) 4550 L CO2 (g) 1L Volume of a cube = side3 Side = 3√V = 3√161 ft3 Side = 5.44 ft (5’ 5”) 3 "Special Cases" or "A gas undergoes a change" problems For a balloon: A gas at a temperature of 98.0 C (371 K) occupies a balloon with a volume of 125.0 mL. To what temperature must the gas be lowered to occupy a volume of 100.0 mL? PV = nRT PV/nT = R = constant P1V1 PV = 2 2 n1T1 n 2T2 P is a constant V is NOT a constant n is a constant V1 V = 2 T1 T2 125.0 mL = 100.0 mL 371 K T2 T2 = 296.8 K or 23.8 ºC We must use K in P1V1 PV = 2 2 n1T1 n 2T2 Why? 4 For a flask: P1V1 PV = 2 2 n1T1 n 2T2 P is a NOT a constant V is a constant n is a constant P1 P = 2 T1 T2 5 Density of gases: What is the density (g/L) of SF6 under standard conditions, 1.00 atm and 273K? Assume one liter of gas (so the mass can be determined for one liter). PV = nRT n = PV/RT = (1.00 atm)(1.00 L)/(0.0821 L•atm/mol•K)(273 K) = 0.0446 moles SF6 146.06 g 0.0446 moles SF6 = 6.51 grams SF6 1mole 6.51 grams is the mass of one liter, so: The density of SF6 is 6.51 g/L. ___ What is the density (g/L) of He under standard conditions, 1.00 atm and 273K? Assume one liter of gas (so the mass can be determined for one liter). PV = nRT n = PV/RT = (1.00 atm)(1.00 L)/(0.0821 L•atm/mol•K)(273 K) = 0.0446 moles He 4.002 g 0.0446 moles SF6 = 0.178 grams He 1mole 0.178 grams is the mass of one liter, so: The density of He is 0.178 g/L. 6 Partial Pressure: A 10.0-L flask at a temperature of 300.0 K contains one mol of O2 gas, 2 moles of CO2 gas, and 7 moles of N2 gas. Calculate the total and partial pressures. PV = nRT P = nRT/V The partial pressure of oxygen (the pressure inside the flask due to the oxygen gas molecules: P(oxygen) = n(oxygen) RT/V P(oxygen) = (1.00 mol)(0.0821 L•atm/mol•K)(300 K)/(10.00 L) = 2.46 atm The partial pressure of carbon dioxide (the pressure inside the flask due to the carbon dioxide gas molecules: P(oxygen) = n(carbon dioxide) RT/V P(oxygen) = (2.00 mol)(0.0821 L•atm/mol•K)(300 K)/(10.00 L) = 4.93 atm The partial pressure of nitrogen (the pressure inside the flask due to the nitrogen gas molecules: P(oxygen) = n(nitrogen) RT/V P(oxygen) = (7.00 mol)(0.0821 L•atm/mol•K)(300 K)/(10.00 L) = 17.2 atm Total Pressure = 2.46 atm + 4.93 atm + 17.2 atm = 24.6 atm 7 Real Gases: Recall for ideal gases: PV=nRT The van der Waals equation for real gases: "a" accounts for the attractive forces between gas particles (a is low for small, non-polar, gases) "b" accounts for the actual gas particle size (b is low for small gases) van der Waals Coefficients Gas a (Pa m3) b(L/mol) Neon 0.0212 0.1710 Hydrogen 0.0245 2.661 Carbon dioxide 0.396 4.269 Water vapor 0.547 3.052 Another, more sophisticated model: 8