Chemical Kinetics : rate of a chemical reaction
advertisement
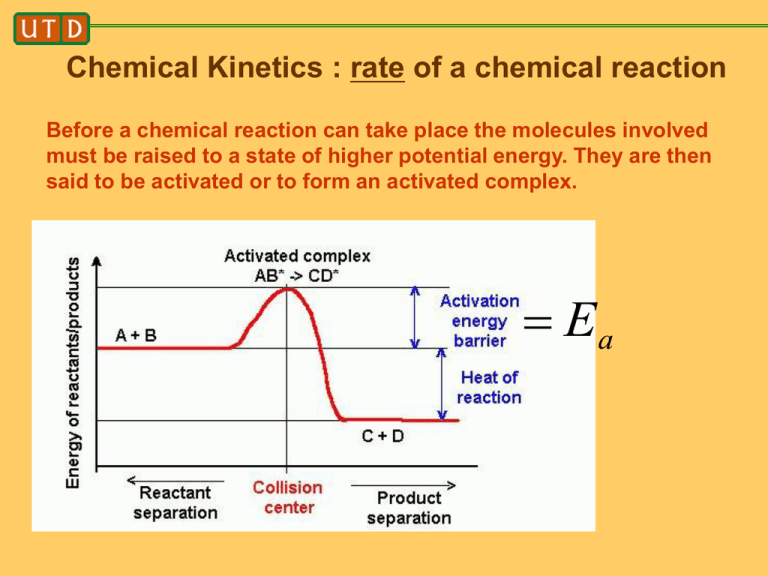
Chemical Kinetics : rate of a chemical reaction Before a chemical reaction can take place the molecules involved must be raised to a state of higher potential energy. They are then said to be activated or to form an activated complex. Ea In 1889 Arrhenius said: 1) van’t Hoff eq. for temperature coefficient of equilibrium constant is 2) d ln K c E 2 dT RT mass-action law relates equilibrium constant to the ratio of rate constants k Kc f kb Hence a reasonable eq. for the variation of rate constant with temperature is d lnk E a 2 dT RT Where Ea is the activation energy of the reaction If Ea does not depend on temperature, we can integrate this last eq. to obtain Ea ln k ln A RT where ln A is the constant of integration. Hence k Ae E a / RT This is the famous Arrhenius eq. for the rate constant. According to Arrhenius, molecules must acquire a certain critical energy Ea before they can react. The Boltzmann factor eE a / RT is the fraction of molecules that manages to obtain the necessary energy. This interpretation is still held to be essentially correct. Henry Eyring (1901-1981) The rate of any chemical reaction can be formulated in terms of its activated complex. The rate of reaction is the number of activated complexes passing per second over the top of the potential energy barrier. This rate is equal to the concentration of activated complexes times the average velocity with which a complex moves across to the product side. Calculation of conc. of activated complexes is greatly simplified if we assume that they are in equil. with the reactants. This equil. can then be treated by means of thermodynamics or statistical mechanics. Transition State Theory Consider this equilibrium: A B AB products equil. constant for the formation of the complex is [AB] K [A][B] the conc. of complexes is thus [AB] K [A][B] according to transition state theory, the rate of reaction is d[A]/dt AB (rate of passage over barrier) The rate of passage over the barrier is equal to the frequency with which the complex flies apart into the products. The complex flies apart when one of its vibrations becomes a translation, and what was formerly one of the bonds holding the complex together becomes simply the line of centers between separating fragments. The frequency is equal to /h where is the average energy of the vibration leading to decomposition. Since by hypothesis this is a thoroughly excited vibration at temperature T, it has its classical energy kT and hence frequency kT/h The reaction rate is therefore d[A] kT k2[A][B] K [A][B] dt h with rate constant kT k2 K h This is the general expression given by transition state theory for the rate constant of any elementary reaction. To be precise, the expression for k2 should be multiplied by a factor called the transmission coefficient, which is the probability that the complex will not recross the transition state and dissociate back into products. In basic TST, 1 The activated complex is similar to a normal stable molecule in every respect save one. The sole difference is that one of its vibrational degrees of freedom is missing, having been transformed into the translation along the reaction coordinate. Instead of 3N-6 vibrational modes, it has 3N-7 modes (non-linear case). We can formulate k2 in thermodynamic terms by introducing the standard free energy change G RT ln K o This is the difference between the free energy of the activated complex and that of the reactants, when all are in their standard states. kT G o / RT kT S o / R H o / RT k2 e e e h h The quantities G o H o S oare called the free energy of activation, the heat of activation, and the entropy of activation. The heat of activation is almost equivalent to the experimental energy of activation Ea, except for a PV term which is negligible for solid or liquid systems. Chemical Before can correct for a wrong choice of transition state this way as well challenges for computational modeling 1) where/what is the transition state? 2) what is the reaction coordinate? Schematic representation of the free energy landscape with two stable, attractive wells separated by a transition state ridge, which connects the highest free energy points of all possible paths connecting the reactant and product states. The dotted line represents a new trajectory that was branched of at point p from an old trajectory (bold line) and surpasses the TS ridge at a lower point. Chemical Chemical Before Chemical Before Chemical Before Chemical Before Chemical Before Chemical Before Example of a complicated reaction coordinate: aqueous proton transfer reaction AH (aq) B (aq) A(aq) BH (aq) what is the reaction coordinate? Chemical Before Transition path sampling Chemical Chemical Chemical