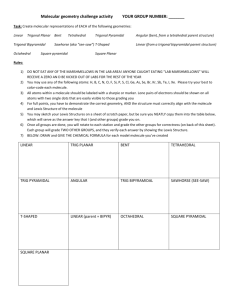
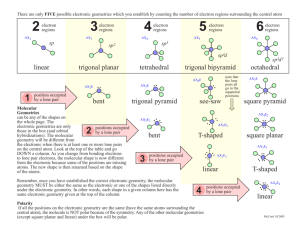
Chemistry Midterm 3 Review Sheet
advertisement
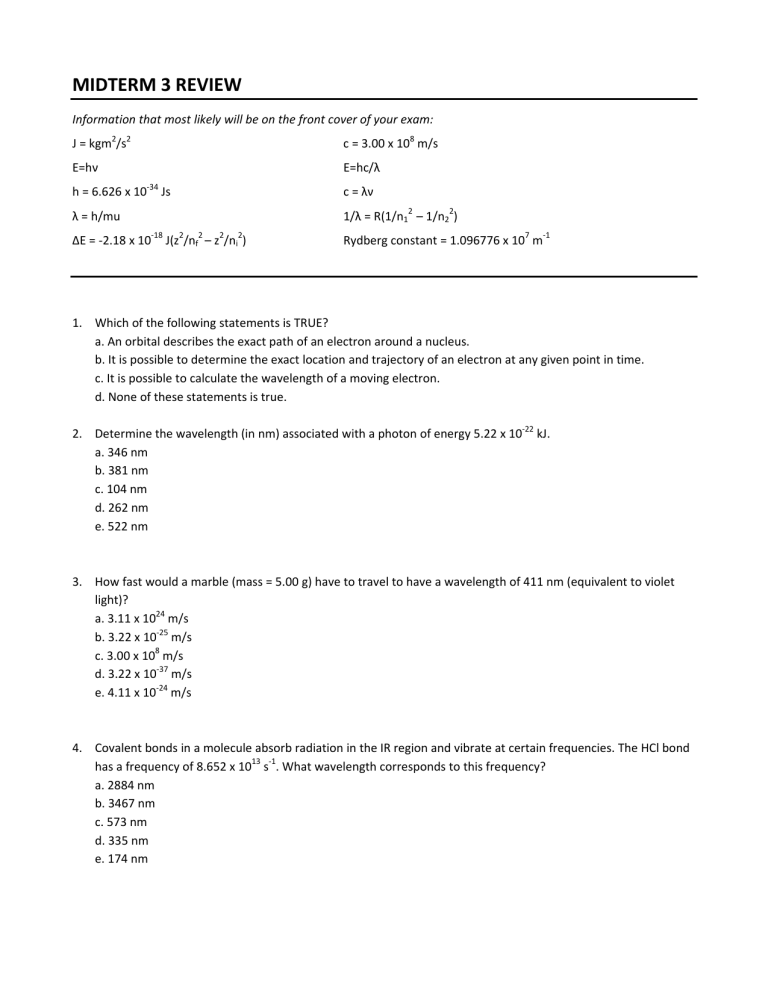
MIDTERM 3 REVIEW Information that most likely will be on the front cover of your exam: J = kgm2/s2 c = 3.00 x 108 m/s E=hν E=hc/λ ‐34 h = 6.626 x 10 Js c = λν λ = h/mu 1/λ = R(1/n12 – 1/n22) ΔE = ‐2.18 x 10‐18 J(z2/nf2 – z2/ni2) Rydberg constant = 1.096776 x 107 m‐1 1. Which of the following statements is TRUE? a. An orbital describes the exact path of an electron around a nucleus. b. It is possible to determine the exact location and trajectory of an electron at any given point in time. c. It is possible to calculate the wavelength of a moving electron. d. None of these statements is true. 2. Determine the wavelength (in nm) associated with a photon of energy 5.22 x 10‐22 kJ. a. 346 nm b. 381 nm c. 104 nm d. 262 nm e. 522 nm 3. How fast would a marble (mass = 5.00 g) have to travel to have a wavelength of 411 nm (equivalent to violet light)? a. 3.11 x 1024 m/s b. 3.22 x 10‐25 m/s c. 3.00 x 108 m/s d. 3.22 x 10‐37 m/s e. 4.11 x 10‐24 m/s 4. Covalent bonds in a molecule absorb radiation in the IR region and vibrate at certain frequencies. The HCl bond has a frequency of 8.652 x 1013 s‐1. What wavelength corresponds to this frequency? a. 2884 nm b. 3467 nm c. 573 nm d. 335 nm e. 174 nm 5. Which of the following one‐electron species will be the easiest (requires least amount of energy) to ionize? a. N6+ b. F8+ c. K18+ d. He+ e. B4+ 6. An electron in the n= 5 level emits a photon with a wavelength of 1282 nm. To what level has the electron moved? a. n = 1 b. n = 2 c. n = 3 d. n = 4 e. n = ∞ 7. Choose the FALSE statement from below. a. It is possible to know the exact location and speed of an electron at any given time. b. Light has been shown to have momentum. c. All matter travels in waves. d. The energy of an atom is quantized because of the wave motion of electrons. e. Orbitals can be described as the small volume around a nucleus where a particular electron has the highest probability of being found. 8. Which of the following frequencies corresponds to light with the longest wavelength? a. 3.88 x 105 Hz b. 4.97 x 1012 Hz c. 6.73 x 107 Hz d. 2.11 x 109 Hz e. 8.45 x 1015 Hz 9. Which of the following transitions in an H atom would have the highest frequency? a. n = 1 to n = 2 b. n = 2 to n = 4 c. n = 3 to n = 4 d. n = 4 to n = 5 e. n = 3 to n = 6 10. Red light (671 nm) is emitted by excited Li atoms. How much energy is emitted by 0.25 moles of Li atoms? a. 44.6 kJ b. 1.33 x 10‐31 kJ c. 2.96 x 10‐19 kJ d. 20.1 kJ e. 7.40 x 10‐20 kJ 11. Determine the set of quantum numbers that describe the electron added to a Br atom as it forms an ion (last electron in the Br– ion). a. n = 4, l = 1, ml = 0, ms = + ½ b. n = 5, l = 4, ml = 3, ms = + ½ c. n = 3, l = 0, ml = 0, ms = – ½ d. n = 4, l = 1, ml = 1, ms = – ½ e. n = 4, l = 2, ml = 2, ms = – ½ 12. Place the following in order of decreasing ionic size. Sr2+ Se2‐ Br– Rb+ a. Sr2+ > Rb+ > Se2‐ > Br– b. Sr2+ > Rb+ > Br– > Se2‐ c. Br– > Se2‐ > Sr2+ > Rb+ d. Rb+ > Br– > Se2‐ > Sr2+ e. Se2‐ > Br– > Rb+ > Sr2+ 13. Which of the following elements have the highest IE2 (second ionization energy)? a. Mg b. Si c. Br d. K e. Ti 14. Determine the identity of the Period 2 element that possesses the following ionization energies (in MJ/mol). IE1= 1.09 IE2= 2.35 IE3=4.62 IE4 =6.22 IE5= 37.83 IE6= 47.28 a. O b. N c. C d. B e. Be 15. Which of the following is paramagnetic? a. Nb3+ b. Ti4+ c. Sr d. Zn e. None of the above. 16. Which of the following will be MOST attracted to a magnetic field (most paramagnetic)? a. Mn b. Co c. Zn2+ d. Sc e. Ar 17. What is the formal charge on the central O in O3? a. ‐2 b. ‐1 c. 0 d. +1 e. +2 18. Determine the best Lewis structure for SO2Cl2. 19. Determine the molecular shape and polarity of BrCl3. a. trigonal planar, nonpolar b. trigonal pyramidal, nonpolar c. trigonal pyramidal, polar d. T shaped, polar e. T shaped, nonpolar 20. Which of the following statements is TRUE concerning the Lewis structure of CF2I2 shown below? a. The shape of this molecule is square planar. b. This molecule is nonpolar. c. There are additional resonance structures that should be shown. d. Each fluorine is attracting electron density away from the carbon. e. All of the above statements are true. 21. How many electrons in a given atom can have the following quantum numbers? n = 3, l = 2 a. b. c. d. e. 2 8 10 18 32 22. How many orbitals in a given atom can be described by the following quantum numbers? n = 4, l = 2 a. b. c. d. e. 3 4 5 6 10 23. Determine the ground state electron configuration for Se+ ion. a. [Ar]4s23d104p5 b. [Ar]4s23d104p4 c. [Ar]4s23d104p6 d. [Ar]4s23d104p3 e. [Ar]4s13d104p4 24. Place the following in order of decreasing size: Mg2+ O2ˉ a. O2ˉ > Mg2+ > Iˉ b. Iˉ > Mg2+ > O2ˉ c. Mg2+ > O2ˉ > Iˉ d. O2ˉ > Iˉ > Mg2+ e. Iˉ > O2ˉ > Mg2+ 25. Which of the following would you predict to have the highest IE2? a. Mg b. K c. Na d. Sr e. Al 26. Which of the following would you predict to be paramagnetic? a. Nb3+ b. Al3+ c. Ti4+ d. Cd2+ e. More than one of these ions are paramagnetic. Iˉ 27. Which of the following is the best Lewis structure for XeO2F2? 28. Draw the Lewis Structures and determine the molecular shape for the following compounds/ions. BrF3 KrCl2 SeS2 BrF5 BrF3 KrCl2 SeS2 BrF5 a. T‐shape linear bent square pyramidal b. trigonal bipyramidal linear linear octahedral c. tetrahedral trigonal planar trigonal bipyramidal d. trigonal planar bent linear square planar e. trigonal planar linear linear trigonal bipyramidal trigonal pyramidal 29. How many of the compounds in question 12 are polar? a. 0 b. 1 c. 2 d. 3 e. 4 30. Which of the following compounds has a square pyramidal molecular shape? a. SO2 b. SCl4 c. BrCl5 d. PF5 e. none of the above 31. The bromate ion, BrO3–, has a molecular shape of _____________ and a formal charge of ______ on the Br. a. trigonal planar, +2 b. trigonal planar, 0 c. trigonal pyramidal, +2 d. trigonal pyramidal, 0 e. tetrahedral, +2 32. Determine the electron geometry and molecular shape for XeF4. a. b. c. d. e. Electron Geometry Octahedral Tetrahedral Octahedral Trigonal Bipyramidal Trigonal Bipyramidal Molecular Shape Square Planar Tetrahedral See‐Saw See‐Saw Tetrahedral 33. Place the following compounds in order of increasing bond angles. SO2 OF2 NCl3 CO2 a. NCl3 < SO2 = CO2 = OF2 b. OF2 < NCl3 < SO2 = CO2 c. SO2 < NCl3 < OF2 < CO2 d. SO2 < OF2 < CO2 < NCl3 e. OF2 < NCl3 < SO2 < CO2 34. Place the following in order of decreasing F‐A‐F bond angles (“A” represents the central atom in each of the following compounds). I. OF2 a. I > II > III b. III > I > II c. III > II > I d. II > I > III e. I = II > III II. SiF4 III. SiF62– 35. Which of the following compounds is polar? SCl4 a. SCl4 b. KrF4 c. SeBr2 d. SCl4 and SeBr2 e. none of the above 36. Which of the following compounds is polar? a. SiO2 b. IF5 c. CF4 d. BF3 e. None of the above are polar. KrF4 SeBr2



![Which is the correct Lewis structure for the nitrate ion, [NO3]– ? a) b](http://s3.studylib.net/store/data/008121614_1-3f41411d21eef682c95d3c7778684719-300x300.png)