Chemistry – Electron Probability
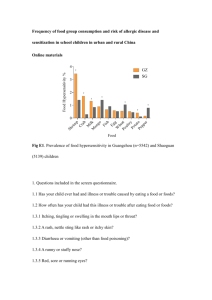
Chemistry
Electron Probability
Visualizing a Probability Region
T
MATERIALS
beans, pinto
cup, 3-oz plastic
graduated cylinder, 100 mL
meter stick
string
tape, masking
target, custom plastic
he picture of an atom that you may have in your mind—that of a nucleus
with electrons orbiting it like planets around the Sun—is outdated and
incorrect. We now know that electrons do not travel in orbits but rather
simply “exist” in regions of space around the atoms. The descriptions and shapes
of these regions are explained by high-level mathematics and physics concepts
that are beyond the scope of this course. However, we can introduce you to
some of the basic probability concepts that underlie these mathematical analyses
through this simple experiment.
Beans will represent an electron and the center of the target will represent the
nucleus. Each bean that lands on the target will represent one possible location
of an electron. By repeating the drop with 200 “electrons,” we begin to get a
statistical picture of the region where there is the greatest probability that the
“electron” will land.
This region of probability is the underlying concept for how we now describe
electrons in atoms. The size of the region will be compared for two different drop
heights, representing two different “energy levels” that might be present in an
atom.
PURPOSE
In this activity, you will drop beans on a target and determine the region with the
greatest probability of hits.
Copyright © 2014 National Math + Science Initiative, Dallas, Texas. All rights reserved. Visit us online at www.nms.org.
1
Chemistry – Electron Probability
PROCEDURE
Work in groups of four.
1. Place the target on the floor and smooth out the wrinkles as much as
possible.
2. Fill a graduated cylinder with beans.
3. Use a meter stick to measure a height approximately 0.5 m from the center of
the target.
4. Using your hand to cover the top of the cylinder of beans, invert the cylinder
so that the opening of the cylinder is at the 0.5 m drop height. Remove your
hand and allow the beans to fall onto the target. Repeat with another cylinder
of beans if needed until approximately 200 beans are on the target.
5. Position a length of string on the target to make a circle such that
approximately 90% of the beans are on the inside of the circle and
approximately 10% of the beans are on the outside of the circle.
This region represents the area of your target where there is a 90%
probability of finding a bean electron.
6. Use the meter stick to measure the average radius of this 90% probability
region, and record this value on your student answer page.
7. Count the number of beans that are in each concentric ring on the target and
record this value in the data table on your student answer page. Count any
beans that landed on a line in the higher numbered area.
8. Repeat Step 4 through Step 7 with a drop height of 1.0 m.
9. When you are finished with both drop heights, clean up your materials as
your teacher instructs.
Copyright © 2014 National Math + Science Initiative, Dallas, Texas. All rights reserved. Visit us online at www.nms.org.
2
Chemistry – Electron Probability
DATA AND OBSERVATIONS
Radius of 90% probability region:
0.5 m drop height = _____________
1.0 m drop height = _____________
Table 1. Data Table
Number of Beans
Area
0.5 m Drop Height
1.0 m Drop Height
1
2
3
4
5
6
Copyright © 2014 National Math + Science Initiative, Dallas, Texas. All rights reserved. Visit us online at www.nms.org.
3
Chemistry – Electron Probability
ANALYSIS
1. Plot the coordinate points (area number, number of beans) for the 0.5 m
drop height on the grid provided. Include (0,0) as one of your points. Draw a
smooth curve that connects all of the points.
Copyright © 2014 National Math + Science Initiative, Dallas, Texas. All rights reserved. Visit us online at www.nms.org.
4
Chemistry – Electron Probability
ANALYSIS (CONTINUED)
2. On the same grid, plot the coordinate points for the 1.0 m drop height.
Include (0,0) as one of your points. Draw a smooth curve that connects all of
the points.
Copyright © 2014 National Math + Science Initiative, Dallas, Texas. All rights reserved. Visit us online at www.nms.org.
5
Chemistry – Electron Probability
CONCLUSION QUESTIONS
1. When dropped from the same height, why don’t all of the beans land in the
same location?
2. As the distance from the center of the target increases, what happens to the
probability of finding a bean? Write a similar statement using the terms
nucleus and electron instead of center and bean.
3. Compare the radius of 90% probability for the 0.5 m and 1.0 m drop heights.
What conclusion can you draw about the size of the 90% probability region
relative to the drop height, or “energy level”?
4. What shape best approximates the pattern made by the beans on the target?
What would this look like in three dimensions rather than two?
Copyright © 2014 National Math + Science Initiative, Dallas, Texas. All rights reserved. Visit us online at www.nms.org.
6