Quantum Numbers (http://chemed.chem.purdue.edu/genchem
advertisement
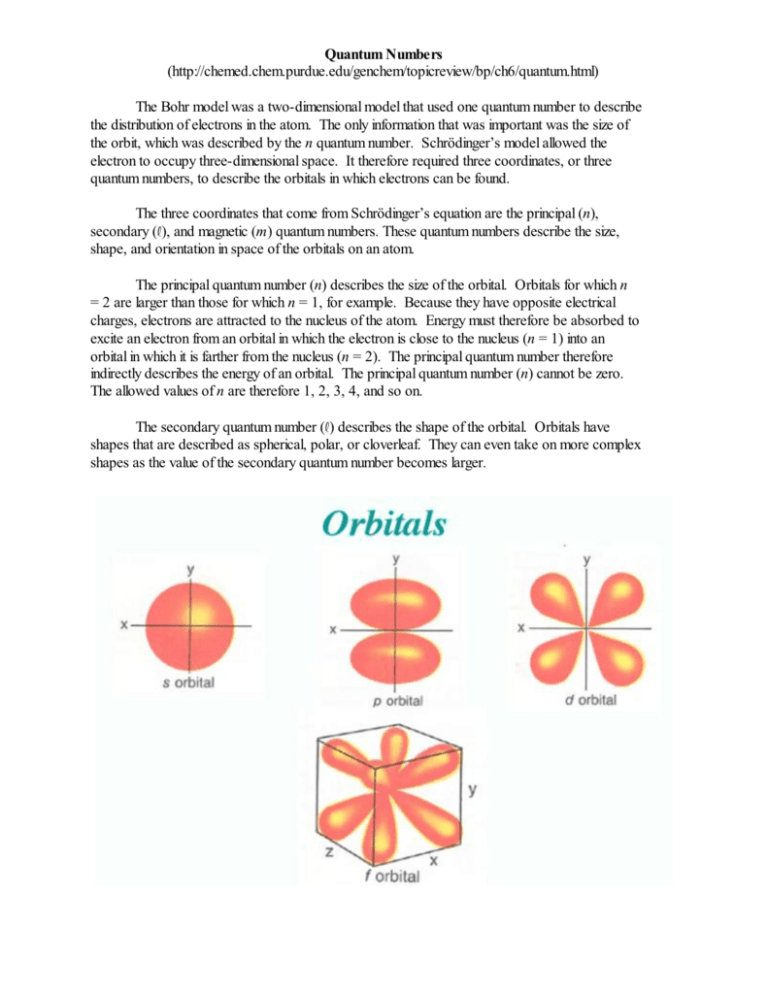
Quantum Numbers (http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch6/quantum.html) The Bohr model was a two-dimensional model that used one quantum number to describe the distribution of electrons in the atom. The only information that was important was the size of the orbit, which was described by the n quantum number. Schrödinger’s model allowed the electron to occupy three-dimensional space. It therefore required three coordinates, or three quantum numbers, to describe the orbitals in which electrons can be found. The three coordinates that come from Schrödinger’s equation are the principal (n), secondary (R), and magnetic (m) quantum numbers. These quantum numbers describe the size, shape, and orientation in space of the orbitals on an atom. The principal quantum number (n) describes the size of the orbital. Orbitals for which n = 2 are larger than those for which n = 1, for example. Because they have opposite electrical charges, electrons are attracted to the nucleus of the atom. Energy must therefore be absorbed to excite an electron from an orbital in which the electron is close to the nucleus (n = 1) into an orbital in which it is farther from the nucleus (n = 2). The principal quantum number therefore indirectly describes the energy of an orbital. The principal quantum number (n) cannot be zero. The allowed values of n are therefore 1, 2, 3, 4, and so on. The secondary quantum number (R) describes the shape of the orbital. Orbitals have shapes that are described as spherical, polar, or cloverleaf. They can even take on more complex shapes as the value of the secondary quantum number becomes larger. There is only one way in which a sphere can be oriented in space. Orbitals that have polar or cloverleaf shapes, however, can point in different directions. We therefore need a third quantum number, known as the magnetic quantum number (m), to describe the orientation in space of a particular orbital. (It is called the magnetic quantum number because the effect of different orientations of orbitals was first observed in the presence of a magnetic field.) Quantum Numbers and Atomic Orbitals (http://www.angelo.edu/faculty/kboudrea/general/quantum_numbers/Quantum_Numbers.htm) By solving the Schrödinger equation, we obtain a set of mathematical equations which describe the probability of finding electrons at certain energy levels within an atom. An atomic orbital describes a region of space in which there is a high probability of finding the electron. Each electron in an atom is described by four different quantum numbers. The first three (n, R, and m) specify the particular orbital of interest, and the fourth (s) specifies how many electrons can occupy that orbital. The principal quantum number (n) has positive integer values from 1 to 8. It specifies the energy of an electron and the size of the orbital (the distance from the nucleus of the peak in a radial probability distribution plot). All orbitals that have the same value of n are said to be in the same shell (energy level). For a hydrogen atom with n = 1, the electron is in its ground state; if the electron is in the n = 2 orbital, it is in an excited state. The secondary quantum number (R) specifies the shape of an orbital with a particular principal quantum number. The secondary quantum number divides the shells (energy levels) into smaller groups of orbitals called subshells (sublevels). Usually, a letter code (s, p, d, or f) is used to identify R to avoid confusion with n. The value of R also has a slight effect on the energy of the subshell; the energy of the subshell increases with R (s < p < d < f). The magnetic quantum number (m) specifies the orientation in space of an orbital of a given energy (n) and shape (R). This number divides the subshell into individual orbitals which hold the electrons. The s subshell has only one orbital, the p subshell has three orbitals, the d subshell has five orbitals, and the f subshell has seven orbitals. The spin quantum number (s) can have values of +½ or -½. It specifies the orientation of the spin axis of an electron. An electron can spin in only one of two directions, clockwise or counterclockwise (sometimes called up and down). The Pauli Exclusion Principle (named for Wolfgang Pauli, who won the Nobel Prize in 1945) states that no two electrons in the same atom can have identical values for all four of their quantum numbers. What this means is that no more than two electrons can occupy the same orbital and that two electrons in the same orbital must have opposite spins. Because an electron spins, it creates a magnetic field which can be oriented in one of two directions. For two electrons in the same orbital, the spins must be opposite to each other; the spins are said to be paired. These substances are not attracted to magnets and are said to be diamagnetic. Atoms with more electrons that spin in one direction than another contain unpaired electrons. These substances are weakly attracted to magnets and are said to be paramagnetic.









