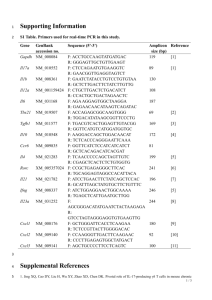
Functional Specialization of Interleukin
advertisement

Immunity Review Functional Specialization of Interleukin-17 Family Members Yoichiro Iwakura,1,5,* Harumichi Ishigame,2 Shinobu Saijo,3 and Susumu Nakae4 1Laboratory of Molecular Pathogenesis, Center for Experimental Medicine and Systems Biology, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo108-8639, Japan 2Department of Immunobiology, Yale University School of Medicine, New Haven, CT 06520, USA 3Department of Molecular Immunology, Medical Mycology Research Center, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba 260-8673, Japan 4Frontier Research Initiative, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo, 108-8639, Japan 5Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency, Saitama 332-0012, Japan *Correspondence: iwakura@ims.u-tokyo.ac.jp DOI 10.1016/j.immuni.2011.02.012 Interleukin-17A (IL-17A) is the signature cytokine of the recently identified T helper 17 (Th17) cell subset. IL-17 has six family members (IL-17A to IL-17F). Although IL-17A and IL-17F share the highest amino acid sequence homology, they perform distinct functions; IL-17A is involved in the development of autoimmunity, inflammation, and tumors, and also plays important roles in the host defenses against bacterial and fungal infections, whereas IL-17F is mainly involved in mucosal host defense mechanisms. IL-17E (IL-25) is an amplifier of Th2 immune responses. The functions of IL-17B, IL-17C, and IL-17D remain largely elusive. In this review, we describe the identified functions of each IL-17 family member and discuss the potential of these molecules as therapeutic targets. Introduction Interleukin-17A (IL-17A), also commonly called IL-17, is produced by the T helper 17 (Th17) subset of CD4+ T cells. The discovery of Th17 cells has been one of the most important advances in T cell immunology since the discovery of Th1 and Th2 cells by Mosmann, Coffman, and their colleagues more than two decades ago (Mosmann et al., 1986). Th17 cells preferentially produce IL-17A, IL-17F, IL-21, and IL-22 (McGeachy and Cua, 2008), whereas Th1 and Th2 cells mainly produce interferon-g (IFN-g) and IL-4, respectively. Recent progress in studies of IL-17A and Th17 cells has revealed important roles for IL-17A in the development of allergic and autoimmune diseases as well as in protective mechanisms against bacterial and fungal infections, functions that were previously believed to be mediated by Th1 or Th2 cells. The Il17a gene, originally called the cytotoxic T lymphocyte associated antigen 8 (Ctla8) gene, was first cloned from a murine cytotoxic T lymphocyte (CTL) hybridoma cDNA library. Murine IL-17A is a 21 kDa glycoprotein containing 147 amino acid residues that shares 63% amino acid identity with human IL-17A (155 amino acids), and both mouse and human IL-17A are secreted as disulfide-linked homodimers. Five additional structurally related cytokines were recently identified: IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25), and IL-17F to form the IL-17 family (Kolls and Lindén, 2004; Weaver et al., 2007) and each additional member of the IL-17 family shares 16%– 50% amino acid identity with IL-17A. Among the family members (Table 1), IL-17A and IL-17F share the highest amino acid sequence identity (50%), whereas IL-17E is the most divergent, with 16% identity to IL-17A. Amino acid similarity is higher in the C terminus and in five spatially conserved cysteine residues, four of which form a cystine knot fold that differs from the canonical cystine knot that is observed in transforming growth factor (TGF)-b, bone morphogenic protein, and nerve growth factor superfamilies as a result of the absence of two cysteine residues (Gerhardt et al., 2009; Hymowitz et al., 2001). The sequences of IL-17B, IL-17C, and IL-17E differ substantially from those of IL-17A and IL-17F in the N terminus, with longer extensions for the former three proteins. Furthermore, IL-17B is secreted as a noncovalent dimer, suggesting that IL-17B, IL-17C, and IL-17E may form a distinct subclass (Gerhardt et al., 2009; Hymowitz et al., 2001). The Il17f gene is closely located to the Il17a gene in both humans and mice (mouse chromosome 1, human chromosome 6, respectively), whereas genes for the other members are located on different chromosomes. The IL-17 receptor (IL-17R) family includes five members (IL-17RA to IL-17RE), which contain such conserved structural characteristics as extracellular fibronectin III-like domains and cytoplasmic similar expression to fibroblast growth factor, IL-17R, and Toll-IL-1R family (SEFIR) domains (reviewed in Gaffen, 2009). Functional receptors for IL-17 family cytokines are thought to consist of homodimers or heterodimers (Figure 1). For example, the heterodimer of IL-17RA and IL-17RC is a receptor for homodimers and heterodimers of IL-17A and IL-17F, whereas the heterodimer consisting of IL-17RA and IL-17RB serves as a receptor for IL-17E. Both IL-17B and IL-17E bind to IL-17RB. IL-17C was recently reported to bind to IL-17RE and activate NF-kB. IL-17RD, also called Sef, is preferentially expressed in endothelial, epithelial, and smooth muscle cells, but not leukocytes. The ligands for this receptor, however, have yet to be identified. Of note, IL-17A, IL-17B, IL-17C, and IL-17F, but not IL-17E, can induce the expression of proinflammatory cytokines such as tumor necrosis factor (TNF) and IL-1b from fibroblasts and peritoneal exudate cells and promote neutrophil migration, suggesting that these family members play similar roles in the Immunity 34, February 25, 2011 ª2011 Elsevier Inc. 149 Immunity Review Table 1. Mouse IL-17 Family Members Percent Homology with IL-17A Percent Homology with Human Ligand Receptor Producer Cells Target Cells Proposed Functions IL-17A (IL-17) IL-17RA and IL-17RC 100 63 CD4+ T cell, CD8+ T cell, gd T cell, NKT cell, LTi-like cell, neutrophil and Paneth cell epithelial cell, fibroblast, keratinocyte, synoviocyte, endothelial cell, T cell, B cell and macrophage proinflammatory cytokine and chemokine induction; neutrophil recruitment; antimicrobial peptide induction; osteoclastogenesis; angiogenesis; promotion of T cell priming and Ab production IL-17B IL-17RB 24 88 chondrocyte and neuron monocyte and endothelial cell proinflammatory cytokine induction; neutrophil recruitment IL-17C IL-17RE 26 83 CD4+ T cell, DC, macrophage and keratinocyte monocyte proinflammatory cytokine induction; neutrophil recruitment IL-17D unknown 30 78 CD4+ T cell and B cell endothelial cell and myeloid progenitor proinflammatory cytokine induction IL-17E (IL-25) IL-17RA and IL-17RB 16 81 CD4+ cell, CD8+ T cell, mast cell, eosinophil, epithelial cell and endothelial cell T cell, macrophage, epithelial cell, NHC, MMPtype2 cell, nuocyte, and Ih2 cell eosinophil recruitment; promotion of Th2 and Th9 cell responses; suppression of Th1 and Th17 cell responses IL-17F IL-17RA and IL-17RC 50 77 CD4+ cell, CD8+ T cell, gd T cell, NKT cell, LTi-like cell, and epithelial cell epithelial cell, fibroblast, keratinocyte, synoviocyte and endothelial cell proinflammatory cytokine and chemokine induction; neutrophil recruitment; antimicrobial peptide induction LTi ; lymphoid tissue inducer, DC; dendritic cell, NHC ; natural helper cell, MMPtype2; multipotent progenitor type2, Ih2; innate type 2 helper. development of certain diseases. Alternatively, IL-17E appears to be involved in promoting Th2 cell-type immune responses. However, the functional roles of other members of the IL-17 family have not been as well characterized as IL-17A. In this review, we will summarize the recent progress of functional studies of these IL-17 family cytokines in diseases and discuss areas for future research. IL-17F is a weaker inducer of proinflammatory cytokine expression and is produced by a wider range of cell types, including innate immune cells and epithelial cells. Moreover, the tissue distribution of IL-17RA and IL-17RC is different. These differences may result in some functional specialization of these cytokines. IL-17A and IL-17F IL-17A and IL-17F are highly homologous and bind to the same receptor. Furthermore, IL-17A and IL-17F can both be secreted as disulfide-linked homodimers or heterodimers. Thus, these two molecules are likely to have similar biological activities (reviewed in Iwakura et al., 2008; Reynolds et al., 2010). Indeed, both IL-17A and IL-17F are involved in the development of inflammation and host defense against infection by inducing the expression of genes encoding proinflammatory cytokines (TNF, IL-1, IL-6, G-CSF, and GM-CSF), chemokines (CXCL1, CXCL5, IL-8, CCL2, and CCL7), antimicrobial peptides (defensins and S100 proteins), and matrix metalloproteinases (MMP1, MMP3, and MMP13) from fibroblasts, endothelial cells, and epithelial cells (Figure 2). IL-17A also promotes SCF- and G-CSF-mediated granulopoiesis and recruits neutrophils to the inflammatory sites. IL-17A also induces the expression of intercellular cell adhesion molecule 1 (ICAM-1) in keratinocytes as well as iNOS and cyclooxygenase-2 in chondrocytes. However, IL-17A and IL-17F Producer Cells IL-17A and IL-17F were initially reported to be predominantly expressed in activated T cells. Linking IL-17A- and IL-17Fproducing CD4+ T cells to IL-23 effector function led to the concept that Th17 cells belong to a distinct CD4+ T cell subset (McGeachy and Cua, 2008). Th17 cell differentiation from naive CD4+ T cells is controlled by several cytokines including TGF-b, IL-6, and IL-21, which activate Stat3- and IRF4-dependent expression of retinoic acid receptor-related orphan receptor-gt (RORgt) (reviewed in Hirahara et al., 2010; Korn et al., 2009; Zhou and Littman, 2009). Other transcription factors, such as RORa, basic leucine zipper transcription factor (Batf), Runx1, and IkBz, also regulate Th17 cell differentiation in cooperation with RORgt. Both IL-1 and IL-23 are also critical for Th17 cell differentiation, growth, survival, and effector functions. In humans, IL-1b, IL-21, IL-23, and TGF-b are required for the development of Th17 cells expressing IL-17A, IL-17F, IL-22, and RORgt, although the requirement for TGF-b in human 150 Immunity 34, February 25, 2011 ª2011 Elsevier Inc. Immunity Review IL-17RC Act1 Act1 TRAF6 ? IL-17RA IL-17B IL-17RB Act1 TRAF3 Unknown TRAF6 TAK1 FnIII ERK MAPK AP-1 IL-17D Act1 TRAF6 TAK1 IL-17C IL-17RD IL-17RA IL-17E (IL-25) Unknown IL-17F IL-17RE IL-17A+F IL-17RB IL-17A MAPK NF-κB C/EBP AP-1 SEFIR NF-κB C/EBP Figure 1. IL-17 and the IL-17 Receptor Families Six IL-17 family cytokines (IL-17A to IL-17F) and five IL-17R family molecules (IL-17RA to IL-17RE) have been identified. After binding of an IL-17A or IL-17F homodimer or heterodimer to IL-17R (the heterodimer of IL-17RA and IL-17RC), Act1 associates with IL-17RA and/or IL-17RC through its SEFIR domains. Subsequently, the complex associates with TRAF6, leading to the activation of NF-kB, MAPK-AP-1, and C/EBP. Downstream of IL-17R, TRAF3 also associates with Act1 to inhibit Act1-TRAF6-mediated activation of transcription factors. Act1-independent ERK activation also contributes to IL-17R signaling via an unknown molecule (?). Similar to signaling via IL-17R, IL-17E (IL-25) binding to IL-25R (heterodimer of IL-17RA and IL-17RB) results in activation of NF-kB, MAPK-AP-1, and C/EBP via recruitment of Act1 and TRAF6. IL-17RB, but not IL-17RA, contains an intracellular TRAF6-binding motif, which activates NF-kB, but not AP-1, through TRAF6 binding. IL-17B and IL-17C bind to IL-17RB and IL-17RE, respectively; however, the downstream signaling pathway is unknown. The ligand(s) for IL-17RD is also unknown. FnIII, fibronectin III-like domain; SEFIR, similar expression to FGF, IL-17R, and Toll-IL-1R family domain. Th17 cell development still remains elusive (reviewed in Korn et al., 2009). The Th17 cell lineage is heterogeneous population. In addition to IL-17A and IL-17F double-positive cells, populations that are only IL-17A or IL-17F positive have been identified. The mechanisms that regulate IL-17A and IL-17F production also differ; IL-17F is expressed earlier than IL-17A during Th17 cell development (Lee et al., 2009). Although underlying molecular mechanisms have not been described, it is likely that several mediators, such as transcription factor or T cell receptor (TCR) signaling, distinctly regulate the production of the cytokines. Indeed, deficiency of RORa selectively reduced IL-17A production (Yang et al., 2008b), and IL-17A expression was more sensitive to the strength of TCR signaling (Gomez-Rodriguez et al., 2009). In addition to Th17 cells, a wide variety of T cells also produce IL-17A and IL-17F. These cytokines are produced by cytotoxic CD8+ T cells (Tc17) under conditions that are similar to those required by Th17 cells, but different from those required by IFN-g producing CD8+ T cells (Tc1). Similarly, distinct populations of gdT (gd-17) cells and NKT (NKT-17) cells produce IL-17A and IL-17F (reviewed in Cua and Tato, 2010). However, IL-23 and IL-1 can directly induce gd-17 cell development in the absence of IL-6 and TCR ligation because, unlike naive CD4+ and CD8+ T cells, these cells constitutively express IL-23R, IL-1R, and RORgt. Likewise, NKT cells produce IL-17A in the presence of IL-1 and IL-23 in combination with TCR stimulation. These two T cell populations (gd-17 and NKT-17) can rapidly produce IL-17A and IL-17F in response to proinflammatory cytokine stimulation and may therefore provide an essential initial source of these two cytokines. More recently, innate lymphoid populations of neutrophils, monocytes, natural killer cells, and lymphoid tissue inducer (LTi)-like cells have been shown capable of rapidly producing IL-17A and IL-17F (Cua and Tato, 2010). In addition, IL-17A is produced by intestinal Paneth cells (Takahashi et al., 2008), whereas IL-17F mRNA, but not IL-17A mRNA, is expressed in colonic epithelial cells (Ishigame et al., 2009), suggesting that IL-17A and IL-17F from nonlymphoid cells may also regulate immune responses. Substantial efforts are underway to clarify the mechanisms that control IL-17A and IL-17F production in these cell types, and the relative contributions of the resulting cytokines in immune responses. Signaling Mechanism of IL-17A and IL-17F Both Il17ra/ and Il17rc/ mice fail to respond to both IL-17A and IL-17F, indicating that both IL-17RA and IL-17RC are Immunity 34, February 25, 2011 ª2011 Elsevier Inc. 151 Immunity Review T cells Th17 Immune cells Tc17 T cell (Priming) Antigen APC γδ-17 TGF-β IL-6 IL-1 IL-23 Disease development B cell (Antibody production) RA, MS, IBD, psoriasis CHS, DTH, onatopic asthma NKT-17 IL-17A Innate immune cells Macrophage (Secrete IL-1, IL-6, TNF, IL-12) Neutrophil recruitment, tissue destruction, neovascularization Tumor Nonimmune cells LTi-like cells Host protection IL-17F Commensal or pathogenic organisms Nonimmune cells Paneth cell Epithelial cell Against fungi, bacteria Epithelial cell, endothelial cell, keratinocyte, synoviocyte, fibroblast (Secrete IL-6, IL-8, TNF, G-CSF, CXCL1, CXCL2, RANKL, MMP, VEGF, antimicrobial peptides) Figure 2. The Roles of IL-17A and IL-17F in the Development of Inflammatory Diseases and host Defense against Pathogens IL-17A and IL-17F are produced by various cell types including T cells, innate immune cells, and nonimmune cells in response to cytokines such as TGF-b, IL-6, IL-1, and IL-23, which are produced by antigen- or pathogen-stimulated antigen presenting cells (APCs). IL-17A and IL-17F activate immune cells such as T cells, B cells, and macrophages to promote T cell priming, Ab production, and proinflammatory cytokine production, and nonimmune cells to induce many proinflammatory mediators such as cytokines, chemokines, MMP, VEGF, RANKL, and antimicrobial peptides. These mediators induce neutrophil recruitment at inflammatory sites, promote local tissue destruction, induce neovascularization in tumors, enhance osteoclastogenesis, and protect from pathogens, resulting in disease development and host protection. IL-17A is mainly involved in autoimmune and allergic responses, tumor development, and host defense against bacterial and fungal infections, whereas IL-17F plays important roles in the host defense against bacteria and inflammation in epithelial tissues. involved in signal transduction (Figure 1) (reviewed in Gaffen, 2009). However, the expression profiles of IL-17RA and IL-17RC are different among tissues and cell types; IL-17RA distributed mostly immune cells, whereas IL-17RC is preferentially expressed in nonimmune cells (Ishigame et al., 2009; Kuestner et al., 2007). In addition, the binding affinities of IL-17A and IL-17F for these receptors are different; IL-17A has higher affinity to IL-17RA, whereas IL-17F has higher affinity to IL-17RC (Hymowitz et al., 2001; Kuestner et al., 2007). Therefore, the distinct functions of IL-17A and IL-17F may reflect, at least in part, differential expression of IL-17RA and IL-17RC, although the precise structure of the receptors for IL-17A and IL-17F still remains to be elucidated. Association between IL-17RA and IL-17RC is not observed on the resting cell surface, and binding of IL-17A to IL-17RA induces recruitment of IL-17RC to form the IL-17RA-IL-17RC complex. The SEFIR domains of both IL-17RA and IL-17RC are required to activate NF-kB, MAPK, and C/EBP pathways in response to IL-17A or IL-17F (Ho et al., 2010; Hu et al., 2010). The SEFIR domain-containing adaptor protein Act1 (also called TRAF3IP3 and CIKS) directly associates with IL-17RA and IL-17RC via interaction with each SEFIR domain, resulting in the recruitment of TRAF6 and TAK1 to activate NF-kB. Cxcl1 mRNA stabilization in response to IL-17A is dependent on Act1, but not TRAF6, whereas ERK activation is Act1 independent. TRAF3 in association with Act1 negatively regulates IL-17A-mediated NF-kB and MAPK activation (Figure 1) (Zhu et al., 2010). IL-17A and IL-17F in Autoimmune Diseases Multiple sclerosis (MS) and rheumatoid arthritis (RA) have long been believed to be a Th1 cell cytokine-mediated autoimmune disease. Yet myelin oligodendrocyte glycoprotein (MOG)152 Immunity 34, February 25, 2011 ª2011 Elsevier Inc. induced experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA) are much more severe in mice lacking IL-12 or IFN-g activity (McGeachy and Cua, 2008), arguing against the importance of Th1 cells in these diseases. Several studies demonstrated that IL-23 rather than IL-12 is important for EAE and CIA development (Cua et al., 2003; Murphy et al., 2003), suggesting involvement of Th17 cells. Moreover, EAE and CIA development are attenuated in Il17a/ mice (Ishigame et al., 2009; Yang et al., 2008a). However, unlike Il23a/ mice, Il17a/ mice still developed substantial inflammation after MOG immunization, suggesting that other IL-23induced mediators, such as IL-17F and IL-22, may also contribute to the development of EAE. IL-17F, however, is not required for EAE and CIA (Haak et al., 2009; Ishigame et al., 2009; Yang et al., 2008a). Il22/ mice have substantially attenuated CIA (Geboes et al., 2009), whereas IL-22 is dispensable for EAE development (Kreymborg et al., 2007). Furthermore, mice deficient for both IL-17A and IL-17F showed no additional suppression of these disorders (Ishigame et al., 2009), suggesting that IL-17F does not have substantial additive, synergistic, or compensatory effects with IL-17A. It was reported that, in addition to Th17 cells, gd-17 cells are involved in the pathogenesis of EAE and CIA (Petermann et al., 2010; Roark et al., 2007). The roles of IL-17A have also been examined in several other RA models. Deficient mice for IL-1 receptor antagonist (Il1rn/), an endogenous antagonist for IL-1a and IL-1b, spontaneously developed chronic inflammatory arthropathy, which was almost completely suppressed in Il17a/ Il1rn/ mice, but only slightly suppressed in Il17f/ Il1rn/ mice (Ishigame et al., 2009). The development of arthritis was also markedly suppressed in Il17a/ HTLV-I transgenic mice (Iwakura et al., 2008). IL-17A plays a pathogenic role in arthritic mice carrying the Y759F Immunity Review mutation in the gp130 subunit of the IL-6R, which disrupts SOCS3-mediated negative feedback (Ogura et al., 2008). IL17A triggers a positive feedback loop of IL-6 signaling, including activation of the NF-kB and Stat3 in fibroblasts, and the development of inflammation. The development of arthritis in SKG mice, which carry a mutation in ZAP70 of the TCR complex, was also suppressed by a lack of IL-17A (Hirota et al., 2007). The contributions of IL-17F to these disease models remain to be elucidated. It is thought that IL-17A causes inflammation by inducing proinflammatory cytokine expression during the effector phase. Neutralizing IL-17A signaling, however, did not affect K/BxN serum-induced arthritis, in which autoantibodies for GPI activate the alternative pathway of the complement activation pathway and FcgRIII on mast cells to induce inflammatory cytokine expression (Jacobs et al., 2009). These results suggest that IL-17A is not required during the effector phase in this setting. Because T cell sensitization and antibody (Ab) production after immunization with type II collagen were substantially reduced in Il17a/ mice (Nakae et al., 2003), IL-17A may induce autoimmunity by directly activating T cells and B cells during the sensitization phase (Figure 2). In line with this idea, IL-17A is required for germinal center formation and class switch recombination by directly acting on B cells (Mitsdoerffer et al., 2010; Wu et al., 2010). A similar pathogenic role for IL-17A has been reported in systemic lupus erythematosus (SLE); IL-17A concentrations are elevated in patients with SLE and IL-17A enhances survival, proliferation, and Ig class switching in B cells (Doreau et al., 2009). However, the mechanism of T cell sensitization has not been elucidated yet. Additionally, Th17 cells also promote osteoclastogenesis as a result of RANKL expression on the cell surface (Sato et al., 2006). The low activity of IL-17F during autoimmune responses remains puzzling, but may relate to weak proinflammatory cytokine-inducing activity (Yang et al., 2008a) and limited distribution of IL-17RC in immune cells (Ishigame et al., 2009; Kuestner et al., 2007). Interestingly, the development of arthritis in Il1rn/, K/BxN, and SKG mice depends on environmental factors, such as the indigenous microbe Lactobacillus bifidus (Abdollahi-Roodsaz et al., 2008), gut-residing segmented filamentous bacteria (SFB) (Wu et al., 2010), and fungi (Yoshitomi et al., 2005). These pathogens induce Th17 cell differentiation, which results in arthritis, suggesting a link between innate immunity and autoimmune diseases. In humans, IL-17A is detected in synovial fluids and synovium from RA patients and induces proinflammatory cytokine production from synoviocytes (Chabaud et al., 1998). IL-17A mRNA is also detected in cerebrospinal fluid mononuclear cells of MS patients, and myelin peptide reactive Th17 cells are enriched in MS patients (Venken et al., 2010). Th17 cells from MS patient also produce IL-22 and IFN-g and preferentially cross the blood brain barrier, suggesting involvement in the pathology (Kebir et al., 2009). Transferring CD4+CD45RBhi T cells into lymphopenic mice provides a model of inflammatory bowel diseases (IBDs). IL-23 is essential for colitis development in this model, whereas neither IL-17A nor IL-17F is required (Izcue et al., 2008; Leppkes et al., 2009). Transferring Il17f/ CD4+CD45RBhi T cells together with anti-IL-17A substantially reduced colitis, suggesting that IL-17A and IL-17F play redundant roles during colitis development (Leppkes et al., 2009). In a recent study, however, mice receiving Il17a/ CD4+ T cells displayed an accelerated wasting disease, which was associated with increased Th1 cell-related cytokine production, suggesting a protective role for IL-17A (O’Connor et al., 2009). Alternatively, colitis was suppressed by the adoptive transfer of CD4+CD45RBhi T cells that were deficient for IFN-g (Iwakura et al., 2008), highlighting the importance of Th1 cells. Thus, both Th1 and Th17 cells are involved in the pathogenesis of IBDs, although uncovering the precise roles of these cells requires further elucidation. Recently, it was reported that Helicobacter hepaticus-induced chronic colitis is associated with IL-23-dependent production of IL-17A and IFN-g in the colon by Thy1+SCA-1+RORgt+IL-23R+ innate lymphoid cells (Buonocore et al., 2010). In humans, Th17 cells are detected in the gut of Crohn’s disease patients and some of these additionally produce IFN-g (Annunziato et al., 2007). CD161+CD4+ T cells in the gut produce IL-17A upon stimulation with IL-23, suggesting the involvement in Crohn’s disease (Kleinschek et al., 2009). IL-17A-producing innate lymphoid cells are also found in patients with IBDs (Buonocore et al., 2010). The potential contribution of IL-17A to a dextran sodium sulfate (DSS)-induced acute colitis model is controversial; one study reported that Il17a/ mice displayed a substantially reduced clinical score (Ito et al., 2008), whereas another study demonstrated that mice lacking IL-17A or given anti-IL-17A showed severe weight loss and colonic epithelial damage (Ogawa et al., 2004; Yang et al., 2008a). Alternatively, Il17f/ mice developed attenuated colonic inflammation, which was associated with reduced chemokine expression (Yang et al., 2008a). Although the reason for the discrepancies in these studies is not known at present, it is possible that intestinal microbial flora may have differed in these studies and affected the results. Interestingly, IL-22 has a protective role in both DSS- and CD4+CD45RBhi T cell-induced colitis (Zenewicz et al., 2008). This protective function of IL-22 in these models appears to be mediated not only by CD4+ T cell-derived IL-22 but also by NK cell-derived IL-22. Psoriasis is an inflammatory epidermal hyperproliferative skin disease. Psoriasis-like epidermal hyperplasia was induced in the ears of wild-type mice injected with IL-23, whereas little hyperplasia was observed in Il17a/ or Il22/ mice (Rizzo et al., 2011). In contrast, psoriasiform dermatitis that spontaneously develops in Il1rn/ mice was neither dependent on T cells nor IL-17A (Nakajima et al., 2010), suggesting an autoinflammatory mechanism. Nonetheless, recent clinical studies suggest the involvement of IL-17A in psoriasis (Hueber et al., 2010). Owing to a lack of spontaneous IL-17A-dependent psoriasis models, the precise roles of IL-17A in this disorder remain obscure. In humans, the expression of proinflammatory cytokines, including IL-17A, IL-22, and IL-23, are elevated in psoriatic skins (Wilson et al., 2007), and the Tc17 and IL-22-producing CD8+ T cells (Tc22) are suggested to be important for pathogenesis (Res et al., 2010). IL-17A in combination with TNF induces genes that are characteristic of psoriasis from human keratinocytes. Polymorphisms in the IL23R gene are associated with psoriasis, suggesting the involvement of IL-23 in psoriasis (Nair et al., 2009). IL-17A and IL-17F may also contribute to type I diabetes mellitus (T1D). In vitro differentiated Th17 cells induce T1D in nonobese diabetic (NOD)-severe combined immunodeficiency (scid) mice (Bending et al., 2009). In this system, however, the Immunity 34, February 25, 2011 ª2011 Elsevier Inc. 153 Immunity Review development of diabetes was dependent on IFN-g, but not IL-17A, suggesting that Th17 cells were reprogrammed into Th1 cells under the lymphopenic conditions. In contrast, adoptive transfer of IL-23-induced OTI Tc17 cells caused IL-17A- and IL-17F-dependent diabetes in recipients that expressed OVA in b cells of the pancreas (Ciric et al., 2009). The progression of diabetes in NOD mice was inhibited by anti-IL17A (Emamaullee et al., 2009), whereas the incidence of hyperglycemia in Il17a/ NOD mice was comparable to that in Il17a+/+ NOD mice (Komiyama et al., 2006). Thus, the precise role of IL-17A and IL-17F in T1D still remains to be elucidated. IL-17A as a Target for Human Autoimmune Diseases As pathogenic roles of IL-17A are suggested in most autoimmune diseases, clinical trials to inhibit Th17 cell development or neutralize IL-17A have been carried out. An IL-6 receptor Ab (tocilizumab, Hoffmann-La Roche and Chugai) is successfully used for the treatment of RA and Crohn’s disease (Patel and Moreland, 2010). Although the precise mechanism of the action of this Ab is unknown, it is possible that the observed therapeutic effects of this Ab are due, at least in part, to blockade of Th17 cell differentiation. A monoclonal Ab (ustekinumab) specific to p40 subunit of IL-12 and IL-23 produced no substantial therapeutic effects in a trial to treat patients with relapsing-remitting MS (Segal et al., 2008). The reason for the discrepancy with the results obtained in mouse EAE model is currently unclear, but improving an Ab delivery system to the central nervous system may be useful. Unlike the results for MS, the Ab to p40 subunit of IL-12 and IL-23 improved symptoms of Crohn’s disease, similar to results obtained by blocking IL-6 or TNF. This Ab also produced a positive result for psoriasis in a phase III trial and was substantially superior to etanercept in another (Griffiths et al., 2010; Leonardi et al., 2008). In addition, a phase II trial using a similar humanized Ab to p40 subunit of IL-12 and IL-23, ABT-874, was also successful (Kimball et al., 2011). Ustekinumab was also effective in a phase II trial for psoriatic arthritis (Gottlieb et al., 2009). IL-1 is also important for Th17 cell differentiation, particularly in humans. An IL-1 receptor antagonist (anakinra) has been successfully used for the treatment of RA (Geyer and Müller-Ladner, 2010). Because IL-1 is pleiotropic, further studies are needed to elucidate the roles of IL-1 in the development of arthritis and Th17 cell differentiation in humans. Recently, a humanized IL-17A Ab, LY2439821, has been developed to treat RA (Genovese et al., 2010). A group of 20 patients was administered once intravenously, and this was followed by an 8 week evaluation period. Another group of 77 patients received the Ab every 2 weeks for a total of five doses with a total evaluation period of 16 weeks. Patients in both groups showed substantial improvement. Another humanized IL-17A Ab, AIN457, has also produced favorable results for the treatment of RA, psoriasis, and uveitis (Hueber et al., 2010). Groups of 36 patients with psoriasis, 52 patients with RA, and 16 patients with uveitis were given a single intravenous shots of AIN457, and this was followed by a 6 week to 16 week evaluation period. In the RA trial, Ab-injected patients showed rapid and substantial improvements as early as a week after the injection. Almost 75% of Ab-treated patients with psoriasis showed improvement of their symptoms in comparison to 10% of 154 Immunity 34, February 25, 2011 ª2011 Elsevier Inc. patients who received a placebo. Patients with uveitis showed an amelioration of symptoms comparable to that observed in patients treated with infliximab. Thus, blocking IL-17A is beneficial for the treatment of certain autoimmune diseases. The effect of blocking other IL-17 family members including IL-17F has yet to be evaluated in human diseases. IL-17A and IL-17F in Allergic Diseases Delayed-type hypersensitivity (DTH) and contact hypersensitivity (CHS)––two T cell-mediated type IV hypersensitive responses––are attenuated in Il17a/ mice, but not Il17f/ mice, suggesting pathological roles of IL-17A in allergic responses. The antigen-specific T cell population was reduced in Il17a/ mice (Ishigame et al., 2009; Nakae et al., 2002), and Tc17 cell-derived IL-17A induced local inflammation during the elicitation phase of CHS (Iwakura et al., 2008). In patients with atopic dermatitis, IL-17A expression increases in local lesions and the Th17 cell population expands in peripheral blood (Koga et al., 2008). IL-17A may be beneficial in this setting because IL-17A is important for the protection against Staphylococcus aureus that aggravates the disease (see below). Yet IL-17A may also promote disease progression by facilitating local inflammation as has been reported for mice lacking filaggrin, an important component of the skin barrier and a predisposing factor for atopic dermatitis (Oyoshi et al., 2009). The pathogenesis of asthma is complex owing to its heterogeneity; e.g., atopic versus nonatopic, eosinophilic versus neutrophilic, and steroid-effective mild asthma versus steroid-resistant severe asthma (Iwakura et al., 2008). Similar to their wild-type littermates, Il17a/, Il17f/, and Il17a/ Il17f/ mice were shown to develop OVA-induced airway eosinophilia (Ishigame et al., 2009; Nakae et al., 2002). One study, however, has suggested that IL-17A and IL-17F contribute positively and negatively, respectively, to this chronic allergic response (Yang et al., 2008a). Alternatively, overexpression of IL-17A and IL-17F in the lungs causes increased proinflammatory cytokine and chemokine expression, resulting in the inflammation associated with neutrophil infiltration (Yang et al., 2008a). IL-17A, but not IL-17F, is required for OVA-induced airway neutrophilia in DO11.10 mice (Ishigame et al., 2009; Nakae et al., 2002) and in mice injected with DO11.10 Th17 cells (Liang et al., 2007). Airway neutrophilia induced by house dust mite, a major asthma allergen, is also dependent on IL-17A (Lajoie et al., 2010). The population of IL-17A-producing Th2 cells, in addition to those of Th2 cells and Th17 cells (Cosmi et al., 2010; Wang et al., 2010b), was preferentially increased in the lungs of patients with atopic asthma as well as of mice treated with certain protease allergens such as Aspergillus-derived proteases and papain and resulted in an influx of inflammatory leukocytes and asthma exacerbation (Wang et al., 2010b). IL-17F, but not IL17A, mediates airway neutrophilia after inhalation of Aspergillus proteases (Yang et al., 2008a), suggesting the involvement of innate immune cell-derived IL-17F in this response. Th17 cells also contribute to airway remodeling and neutrophilia during chronic airway inflammation (Wang et al., 2010a). Overall, these data suggest that IL-17A and IL-17F are involved in airway neutrophilia, but not eosinophilia, during allergic asthma. The detailed functional differences between IL-17A and IL-17F as Immunity Review well as the roles of Th17 and IL-17A-producing Th2 cells in asthma development remains to be elucidated. IL-17A and IL-17F in Other Immune Cell-Mediated Diseases IL-17A-induced neutrophil recruitment to inflamed sites contributes to various diseases. For example, IL-17A is crucial for neutrophil-mediated ischemia-reperfusion injury in the brain (Shichita et al., 2009). NKT cell-derived IL-17A plays a role in ozone-induced airway neutrophilia, a form of asthma independent of adaptive immunity (Pichavant et al., 2008). IL-17A, which may be produced by gd T cells, is also involved in pulmonary fibrosis and neutrophilia induced by bleomycin (Wilson et al., 2010). Also, like many cytokines, IL-17A may play a role in sepsis (Flierl et al., 2008; Freitas et al., 2009). Rats transferred with soluble IL-17RA, but not Il17a/ mice, showed reduced acute rejection of cardiac transplants (Gorbacheva et al., 2010; Li et al., 2006). This apparent discrepancy suggests that IL-17F and/or IL-17E may also be involved in these responses. IL-17A-mediated neutrophil recruitment accelerates minor histocompatibility antigen-mismatched skin allograft survival (Vokaer et al., 2010), yet IL-17A deficiency stimulates corneal allograft rejection by promoting Th2 cell responses (Cunnusamy et al., 2010). The role of IL-17A in graft-versus-host disease is controversial; IL-17A has been shown to be protective (Yi et al., 2008), pathogenic (Kappel et al., 2009), and nonessential (Nakae et al., 2002), depending on the experimental protocols. IL-17F has not been examined in these diseases. IL-17A and IL-17F in Tumor Development Th17 cells infiltrate into tumor sites and draining lymph nodes in cancer patients (reviewed in Zou and Restifo, 2010). The number of IL-17A-producing cells correlates with poor survival in patients with hepatocellular carcinoma, whereas the number was decreased in patients with advanced ovarian cancer, lung cancer, or non-Hodgkin’s lymphoma. Thus, Th17 cells and IL-17A may play different roles depending on the tumor type and stage. Transplantation of IL-17A-overexpressing tumor cells into immunodeficient mice induced angiogenesis through induction of vascular endothelial growth factor (VEGF) expression, resulting in enhanced tumor growth (Numasaki et al., 2003). T cellderived IL-17A also dramatically increases the release of angiogenic and chemoattractive IL-8 from tumor cells (Tartour et al., 1999). Delayed growth of subcutaneously transplanted B16 melanoma cells and MB49 bladder carcinoma cells was observed in Il17a/ mice, whereas Ifng/ mice showed accelerated growth and augmented IL-17A production in the tumor (Wang et al., 2009). In this case, IL-6 was expressed in response to IL-17A-activated Stat3 in the tumor cells, upregulating the expression of prosurvival and proangiogenic genes. IL-17A is also involved in the development of colonic cancer in multiple intestinal neoplasia (Min) mice, a model of familial adenomatous polyposis (Chae et al., 2010). Interestingly, enterotoxigenic Bacterioides fragilis (ETBF), an intestinal commensal bacteria, can accelerate colonic inflammation and tumor formation in Min mice (Wu et al., 2009). In this model, ETBF selectively induced Th17 cell differentiation via Stat3 activation, and blocking IL-17A as well as IL-23R dramatically reduced tumor devel- opment, indicating that a Stat3- and Th17 cell-dependent pathway contributed to the disease. Th17 cells have also been shown to inhibit tumor development. Lung metastasis of B16-F10 melanoma was increased in Il17a/ mice, and adoptive transfer of tumor-specific Th17 cells prevented tumor development associated with an activation of tumor-specific CD8+ T cells (Martin-Orozco et al., 2009). Tc17 cells also suppressed established B16-F10 melanomas (Hinrichs et al., 2009). Il17a/ mice showed increased tumor growth and metastasis of MC38 cells in lung and subcutaneous tissues, which was associated with reduced IFN-g-producing NK and CD8+ T cells (Kryczek et al., 2009). These results suggest that IL-17A indirectly stimulates antitumor immunity by promoting type 1 immune responses. Thus, IL-17A has both protumor and antitumor activity, depending on the types and stages of tumors. Although the roles of other IL-17 family members including IL-17F in tumor development have not been well described, their potential contributions deserve consideration, particularly in light of their distinct expression profiles. The effects of blocking IL-17A or other IL-17 family members on tumor growth in humans have yet to be examined. IL-17A and IL-17F in Host Defense against Infection In contrast to pathogenic roles of Th17 cytokines in autoimmune diseases, Th17 cytokines protects hosts from pathogens at epithelial and mucosal tissues including the skin, lung, and intestine. Both IL-17A and IL-17F enhance protective immune responses by inducing the production of CXC chemokines, G-CSF, and antimicrobial peptides in epithelial cells and keratinocytes. Indeed, studies using cytokine- and receptor-deficient mice showed that IL-17A and IL-17F were required for responses to extracellular bacterium Klebsiella pneumoniae in the lungs, Citrobacter rodentium in the colon, and Staphylococcus aureus in the skin (Aujla et al., 2008; Ishigame et al., 2009). Interestingly, Il17a/Il17f/ mice were highly susceptible to spontaneous S. aureus infections compared with Il17f/ or Il17a/ mice, and Il17ra/ mice showed higher susceptibility to K. pneumoniae infection than Il17a/ mice, suggesting that IL-17A and IL-17F have overlapping roles in these models. On the other hand, Il17a/ mice, Il17f/ mice, and Il17a/Il17f/ mice were similarly susceptible to C. rodentium (Ishigame et al., 2009). The mechanisms underlying differential responses to S. aureus and C. rodentium are not yet known. A recent study using Il22/ and Il17rc/ mice claims that IL-22, but not IL-17A and IL-17F, is essential for the early host response against C. rodentium (Zheng et al., 2008). IL-22 is produced by innate immune cells, including dendritic cells (DCs) and LTi-like cells during C. rodentium infection and induces the expression of Reg family antimicrobial proteins in colonic epithelial cells (Sonnenberg et al., 2011; Zheng et al., 2008). IL-17A is also required for host defense mechanisms against intracellular pathogens in mice. Although Il17ra/ mice were not more susceptible to Mycobacterium tuberculosis infection (Aujla et al., 2008), the IL-23-IL-17A pathway was required for Th1 cell-type immune responses that protected the host against the intracellular pathogen Francisella tularensis (Lin et al., 2009). In humans, hyper IgE syndrome patients, in which Th17 cell differentiation is suppressed by a STAT3 mutation, are highly susceptible to S. aureus, Candida albicans, and Streptococcus Immunity 34, February 25, 2011 ª2011 Elsevier Inc. 155 Immunity Review pneumoniae infection, resulting in the skin and lung inflammation (reviewed in Milner et al., 2010). Furthermore, preferential depletion of Th17 cells in the intestine dramatically increases the frequency of bacteremia, including nontyphoidal Salmonella serotypes, in monkeys infected with simian immunodeficiency virus, suggesting that a loss of Th17 cells may also cause increased susceptibility to Salmonella in HIV-infected people (Raffatellu et al., 2008). Therefore, in contrast to relatively minor contribution of IL-17F to autoimmune and allergic diseases, both IL-17A and IL-17F play protective roles for the defense against some extracellular bacteria and fungi in the skin and mucoepithelial tissues. This may reflect the fact that IL-17F is produced not only by Th17 cells and innate immune cells but also by epithelial cells and that IL-17RC distributes widely in nonlymphoid tissues making a contrast to IL-17RA, although the immune-activating activity of IL-17F is relatively low. IL-17A is produced rapidly after microbial infection, and gd T cells have been identified as a primary source during early infections with several types of bacteria, including Listeria monocytogenes and Escherichia coli infection (Hamada et al., 2008; Shibata et al., 2007). These studies demonstrated that a lack of IL-17A resulted in increased bacterial burden, suggesting that gd T cell-derived IL-17A promotes neutrophil accumulation to eradicate bacteria. Although NKT cells, LTi-like cells, epithelial cells, and Paneth cells can also produce IL-17A and/or IL-17F, the cell populations responsible for activating antibacterial immunity depending on the pathogen, infected tissue, and time after infection are unknown. Th17 cells are enriched in the gastrointestinal tract under steady-state conditions, and intestinal Th17 cell development is largely dependent on commensal bacteria SFB (GaboriauRouthiau et al., 2009; Ivanov et al., 2009; Wu et al., 2010). Adenosine 50 -triphosphate derived from commensal bacteria or SFB-induced serum amyloid A stimulates DCs to produce Th17 cell-inducing cytokines, including IL-6, IL-23, and TGF-b. Notably, mice lacking Th17 cells because of the absence of SFB showed increased susceptibility to C. rodentium (Ivanov et al., 2009). Thus, intestinal commensal bacteria mediate the development of Th17 cells as well as other IL-17A- and IL-17Fproducing cells, resulting in various effects on host immune responses. Il23a/ and Il17ra/, but not Il12a/, mice are highly susceptible to oral candidiasis because of defective neutrophil recruitment and antimicrobial peptide production (Conti et al., 2009). Il22/ mice are only mildly susceptible to oral candidiasis, suggesting that IL-22 is dispensable for the host defense in this model. IL-17A plays more important role than IL-17F in systemic infection of C. albicans because only Il17a/ mice, but not Il17f/ mice, show increased susceptibility (Saijo et al., 2010). Interestingly, Th17 cell differentiation was strongly induced in naive CD4+ T cells cultured in C. albicans-stimulated bone marrow (BM)-derived DC (BMDC)-conditioned medium, an effect mediated by high concentrations of IL-1 and IL-23 in the medium. Th17 cell differentiation was substantially reduced when dectin-2-deficient BMDC-conditioned medium were used instead, indicating that dectin-2, the receptor for a fungal cell wall component a-mannan, plays a major role for the differentiation of Th17 cells upon infection with C. albicans (Saijo et al., 2010). Similarly, stimulation of dectin-1, the receptor for another 156 Immunity 34, February 25, 2011 ª2011 Elsevier Inc. fungal cell wall component b-glucan, also induced Th17 cell differentiation (LeibundGut-Landmann et al., 2007). IL-17A may inhibit fungal growth by recruiting neutrophils, inducing b-defensins, and activating acquired immune system. IL-17E IL-17E enhances Th2 cell immune responses by inducing Th2 cell cytokines such as IL-4, IL-5, and IL-13 in auxiliary cells and induces IgE production and eosinophilia, contributing to the host defense against nematodes and allergic disorders (Figure 3). The heterodimer consisting of IL-17RA and IL-17RB serves as the receptor for IL-17E (Figure 1) (reviewed in Gaffen, 2009). Although IL-17E binds only IL-17RB, but not IL-17RA, both Il17rb/ and Il17ra/ mice fail to respond to IL-17E, suggesting that both chains are involved in signal transduction. Like IL-17RA, Act1 is recruited to the SEFIR domain of IL-17RB through the interaction of SEFIR between IL-17RB and Act1 after IL-17E bindings (Swaidani et al., 2009). In contrast to IL-17RA, the cytoplasmic TRAF6-binding motif of IL-17RB directly associates with TRAF6 irrespective of IL-17E binding and activates NF-kB upon IL-17E binding. IL-17E is produced by Th2 cells, cecal patch CD4+ and CD8+ T cells, mast cells, and eosinophils (reviewed in Reynolds et al., 2010). Alveolar macrophages and endothelial and epithelial cells may also produce IL-17E in rodents, and skin-resident and monocyte-derived DCs as well as basophils produce IL-17E in humans. IL-17E promotes Th2 and Th9 cells activation, whereas IL-17E can induce IL-5- and IL-13-mediated eosinophilia independently of T cells. Regarding the latter cases, recently identified IL-17E-responsive novel innate cell populations such as natural helper cells (NHCs), multipotent progenitor type2 (MMPtype2) cells, nuocytes, and innate type 2 helper (Ih2) cells are focused on the T cell-independent Th2-type immunity (Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b). Thus, IL-17E is considered to be crucial for both acquired and innate immune responses. IL-17E in Chronic Inflammatory Diseases IL-17E ameliorates autoimmune diabetes (Emamaullee et al., 2009). Like Ifng/ mice, IL-17E deficient (Il25/) mice were susceptible to EAE, which was accompanied by increased number of Th17 cells, whereas IL-17E suppressed EAE even in Ifng/ mice by reducing the Th17 cell population in a manner dependent on IL-13-IL-4Ra signaling (Kleinschek et al., 2007). IL-17E attenuates peptideglycan (PGN)-induced, as well as DSS-, 2, 4, 6-trinitrobenzenesulphonic acid (TNBS)-, and oxazolone-induced colitis, independently of IL-13 (Caruso et al., 2009; Mchenga et al., 2008). IL-17E, which is expressed in epithelial cells and macrophage-like cells of IBD patients, suppressed IL-12p40 production in lipopolysaccharide (LPS)- and PGNstimulated mucosal CD14+ cells from IBD patients (Caruso et al., 2009); this helped to ameliorate certain types of colitis by reducing production of IFN-g (Figure 3). IL-17A- and IL-17Fproducing mast cells and neutrophils, but not T cells, and IL-17E-expressing smooth muscle, endothelial and B cells are suggested to be involved in the pathogenesis of atherosclerosis (de Boer et al., 2010). IL-17E activates IL-17RB+ NKT cells, IL-17RB+ CD11blo CD11c+ F4/80+ macrophages, and airway epithelial cells to secrete Th2 cell cytokines, resulting in airway eosinophilia Immunity Review Antigen and commensal or pathogenic organisms Figure 3. The Roles of IL-17E in the Development of Inflammatory Diseases and Host Defense against Pathogens T cells NKT (IL-4, IL-13) Th2 (IL-4, IL-5, IL-13) Immune cells Th9 (IL-9) Th2 cytokines Macrophage Innate immune cells DC IL-17E T cell Promote: Atopic asthma Eosinophil Macrophage, MPP-type 2 cell, Ih2 cell, NHC, nuocyte (IL-4, IL-5, IL-13) Mast cell Basophil Non-immune cells Non-immune cells Paneth cell Epithelial cell Epithelial cell (IL-4, IL-5, CCL11) (Swaidani et al., 2009; Terashima et al., 2008). Airway eosinophilia was substantially decreased in mice treated with anti-IL17E or soluble IL-17RB-Fc proteins (Ballantyne et al., 2007; Tamachi et al., 2006) as well as in Il25/ mice because of impaired Th2 cell and Th9 cell activation (Angkasekwinai et al., 2010). On the other hand, airway eosinophilia induced by protease(s) from Aspergillus oryzae was only partially reduced in Il25/ mice (Angkasekwinai et al., 2010). Thus, the roles of IL-17E in airway inflammation are clearly different from those of IL-17A and IL-17F, which are involved in airway neutrophilia, but not in eosinophilia (Figure 3). Consistent with this notion, Il17ra/ mice, but not Il17a/ Il17f/ mice, are resistant to eosinophilic acute airway inflammation induced by OVA plus alum (SchnyderCandrian et al., 2006). IL-17E in Tumor Development Administration of IL-17E to mice transplanted with various types of tumor cell lines inhibited tumor growth. The antitumor effect of IL-17E was abolished in scid mice, but not in nude mice, suggesting the involvement of IL-17E-mediated B cell activation (Benatar et al., 2010). However, the role of IL-17E in tumorigenesis is poorly understood. IL-17E in Host Defense against Infection IL-17E is important for the protection against nematodes such as Nippostrongylus brasiliensis and Trichuris muris by promoting Th2 cell cytokine production (Figure 3) (Fallon et al., 2006; Prevent: Helminth infection Block: Th1 and Th17 cell responses IL-17E is crucial for both acquired and innate immune responses. Upon antigen or pathogen stimulation, various cells, such as T cells, innate immune cells, and nonimmune cells, produce IL-17E. IL-17E activates NKT cells, Th2 cells, and Th9 cells to produce Th2 cytokines such as IL-4, IL-5, and IL-13. Activated Th2 cells also produce IL-17E, and it may further amplify T cell-mediated acquired immune responses. IL-17E also enhances type 2 immunity by directly acting on innate immune cells, such as macrophages, multipotent progenitor type2 (MMPtype2) cells, innate type 2 helper (Ih2) cells, natural helper cells (NHCs), and nuocytes, which are the important innate source of Th2 cytokines. In addition, IL-17E promotes epithelial cell secretion of IL-4 and IL-5, and chemokines such as CCL11 to recruit eosinophils. These IL-17E-indeced Th2 cytokines promote allergic disease development as well as host protection against parasites. By contrast, IL-17E directory and indirectory regulates autoimmunity by suppressing Th1 and Th17 responses. Owyang et al., 2006; Zhao et al., 2010). Recent studies have identified innate immune cell populations such as NHC, MMPtype2 cells, nuocytes, and Ih2 cells, which can promote Th2 cell responses in response to IL-17E (Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b). Although the cell surface phenotype and anatomical location are different among these cell populations, they are elicited in the absence of the adaptive immune system (reviewed in Saenz et al., 2010a). The linage relationship between NHCs, MMPtype2 cells, nuocytes, and Ih2 cells, and the contributions of these innate populations in other Th2 cell-related diseases remain to be elucidated. It is also interesting to investigate the relationship between non-B-non-T Lyn innate lymphoid cells that produce IL-17A and IFN-g in response to IL-23 (Buonocore et al., 2010) and those cells that produce Th2 cell cytokines in response to IL-17E. IL-17E produced by intestinal epithelial cells in response to commensal bacteria limits intestinal Th17 cell expansion by inhibiting IL-23 production from macrophages (Zaph et al., 2008). Thus, IL-17E may be involved in Th17 cell-mediated host defense mechanisms against bacteria. Further investigations are needed to discriminate the roles of this cytokine from other Th2 cytokines. IL-17B, IL-17C, and IL-17D IL-17B, IL-17C, and IL-17D were identified through database searches a decade ago. Although these cytokines have similar ability to induce inflammatory mediators as IL-17A and IL-17F, their roles in the immune system remain largely unknown. Although low IL-17B mRNA is detected in several organs, its expression is high in chondrocytes and neurons (Lee et al., 2001; Moseley et al., 2003; Shi et al., 2000). Like IL-17E, IL-17B binds to IL-17RB with lower affinity than IL-17E (Figure 1) Immunity 34, February 25, 2011 ª2011 Elsevier Inc. 157 Immunity Review (reviewed in Gaffen, 2009); however, the signal transduction mechanisms of IL-17B-IL-17RB are unknown. IL-17C is expressed in CD4+ T cells, DCs, and macrophages at inflammatory sites, but not in most normal tissues (Hwang and Kim, 2005; Li et al., 2000; Yamaguchi et al., 2007). IL-17C binds to IL-17RE and activates NF-kB (Gaffen, 2009; Starnes et al., 2002). Similar to IL-17B, IL-17D mRNA is detected in several tissues, whereas, in immune cells, its expression is observed only in resting CD4+ T and B cells (Starnes et al., 2002). The receptor(s) for IL-17D has not yet been identified. Biological Functions of IL-17B, IL-17C, and IL-17D Both IL-17B and IL-17C induce TNF and IL-1b expression from a monocytic cell line and cause neutrophil infiltration (Li et al., 2000; Shi et al., 2000). Elevated expression of IL-17B and IL-17C was observed in local lesions of CIA. Adoptive transfer of Il17b- or Il17c-gene-transduced CD4+ T cells exacerbated CIA accompanied with increased TNF production, whereas the disease was ameliorated in mice treated with IL-17B neutralizing Ab (Yamaguchi et al., 2007). IL-17D, which is most homologous to IL-17B, also induces the expression of IL-6, IL-8, and GM-CSF in endothelial cells (Starnes et al., 2002), and inhibits hematopoietic progenitor colony formation, an activity shared by IL-17A, IL-17F and IL-17E (Broxmeyer et al., 2006). Although the expression of IL-17D has been detected in rheumatoid nodules (Stamp et al., 2008), potential pathogenic roles remain to be elucidated. IL-17C mRNA expression was upregulated in cultured epidermal keratinocytes subjected to S. aureus colonization (Holland et al., 2009). Mycoplasma pneumoniae and the TLR5 agonist flagellin also induce IL-17C expression in lung and gut tissues (Van Maele et al., 2010; Wu et al., 2007). Therefore, IL-17B, IL-17C, and IL-17D may have similar activity to induce inflammatory mediators, and contribute to inflammatory responses like IL-17A and IL-17F. Future experiments using cytokine-blocking Abs or cytokine gene-targeted mice may help to understand the functions of these cytokines in immune responses. Concluding Remarks Clinical studies have shown that blocking the activity of IL-12 and IL-23 (p40), IL-1, or IL-6, which are important for the development of Th17 cells and possibly innate IL-17A-producing cells, is effective for the treatment of inflammatory diseases, such as RA, MS, IBDs, and psoriasis. More recent clinical trials have demonstrated that anti-IL-17A therapies may also be effective to treat some of these diseases. However, because IL-17A plays important roles in the host defense against pathogens, treatments using anti-IL-17A may also increase the risk of opportunistic infections, as have been observed with therapies targeting other cytokines. Thus, clinical application of these approaches requires caution, with particular attention paid to staphylococcus and candida infections. Because IL-17F is often functionally redundant with IL-17A in host defenses against infections and shows relatively lower proinflammatory activity, anti-IL-17A treatment may be safer than other biological therapies. Nevertheless, for some pathogens both IL-17A and IL-17F are required for the eradication. Because other IL-17 family members, especially IL-17E, are also proinflammatory and may be involved in host defense responses, an understanding of the functions of these cytokines will allow the 158 Immunity 34, February 25, 2011 ª2011 Elsevier Inc. development of more effective treatments for allergic and autoimmune disorders and tumors without compromising host defense activity. ACKNOWLEDGMENTS We thank C. Cheng-Mayer for critical reading of the manuscript. This work is supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, CREST, and the Promotion of Basic Research Activities for Innovative Biosciences program (Y.I.) and the Program for Improvement of Research Environment for Young Researchers, The Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology, Japan (S.N.). REFERENCES Abdollahi-Roodsaz, S., Joosten, L.A., Koenders, M.I., Devesa, I., Roelofs, M.F., Radstake, T.R., Heuvelmans-Jacobs, M., Akira, S., Nicklin, M.J., Ribeiro-Dias, F., and van den Berg, W.B. (2008). Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216. Angkasekwinai, P., Chang, S.H., Thapa, M., Watarai, H., and Dong, C. (2010). Regulation of IL-9 expression by IL-25 signaling. Nat. Immunol. 11, 250–256. Annunziato, F., Cosmi, L., Santarlasci, V., Maggi, L., Liotta, F., Mazzinghi, B., Parente, E., Filı̀, L., Ferri, S., Frosali, F., et al. (2007). Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204, 1849–1861. Aujla, S.J., Chan, Y.R., Zheng, M., Fei, M., Askew, D.J., Pociask, D.A., Reinhart, T.A., McAllister, F., Edeal, J., Gaus, K., et al. (2008). IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14, 275–281. Ballantyne, S.J., Barlow, J.L., Jolin, H.E., Nath, P., Williams, A.S., Chung, K.F., Sturton, G., Wong, S.H., and McKenzie, A.N. (2007). Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 120, 1324–1331. Benatar, T., Cao, M.Y., Lee, Y., Lightfoot, J., Feng, N., Gu, X., Lee, V., Jin, H., Wang, M., Wright, J.A., and Young, A.H. (2010). IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo. Cancer Immunol. Immunother. 59, 805–817. Bending, D., De La Peña, H., Veldhoen, M., Phillips, J.M., Uyttenhove, C., Stockinger, B., and Cooke, A. (2009). Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J. Clin. Invest. 119, 565–572. Broxmeyer, H.E., Starnes, T., Ramsey, H., Cooper, S., Dahl, R., Williamson, E., and Hromas, R. (2006). The IL-17 cytokine family members are inhibitors of human hematopoietic progenitor proliferation. Blood 108, 770. Buonocore, S., Ahern, P.P., Uhlig, H.H., Ivanov, I.I., Littman, D.R., Maloy, K.J., and Powrie, F. (2010). Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375. Caruso, R., Sarra, M., Stolfi, C., Rizzo, A., Fina, D., Fantini, M.C., Pallone, F., MacDonald, T.T., and Monteleone, G. (2009). Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology 136, 2270–2279. Chabaud, M., Fossiez, F., Taupin, J.L., and Miossec, P. (1998). Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J. Immunol. 161, 409–414. Chae, W.J., Gibson, T.F., Zelterman, D., Hao, L., Henegariu, O., and Bothwell, A.L. (2010). Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc. Natl. Acad. Sci. USA 107, 5540–5544. Ciric, B., El-behi, M., Cabrera, R., Zhang, G.X., and Rostami, A. (2009). IL-23 drives pathogenic IL-17-producing CD8+ T cells. J. Immunol. 182, 5296–5305. Conti, H.R., Shen, F., Nayyar, N., Stocum, E., Sun, J.N., Lindemann, M.J., Ho, A.W., Hai, J.H., Yu, J.J., Jung, J.W., et al. (2009). Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206, 299–311. Immunity Review Cosmi, L., Maggi, L., Santarlasci, V., Capone, M., Cardilicchia, E., Frosali, F., Querci, V., Angeli, R., Matucci, A., Fambrini, M., et al. (2010). Identification of a novel subset of human circulating memory CD4+ T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 125, 222–230, e221-224. Cua, D.J., and Tato, C.M. (2010). Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489. 12/23 monoclonal antibody, for psoriatic arthritis: Randomised, double-blind, placebo-controlled, crossover trial. Lancet 373, 633–640. Griffiths, C.E., Strober, B.E., van de Kerkhof, P., Ho, V., Fidelus-Gort, R., Yeilding, N., Guzzo, C., Xia, Y., Zhou, B., Li, S., et al; ACCEPT Study Group. (2010). Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N. Engl. J. Med. 362, 118–128. Cua, D.J., Sherlock, J., Chen, Y., Murphy, C.A., Joyce, B., Seymour, B., Lucian, L., To, W., Kwan, S., Churakova, T., et al. (2003). Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748. Haak, S., Croxford, A.L., Kreymborg, K., Heppner, F.L., Pouly, S., Becher, B., and Waisman, A. (2009). IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 119, 61–69. Cunnusamy, K., Chen, P.W., and Niederkorn, J.Y. (2010). IL-17 promotes immune privilege of corneal allografts. J. Immunol. 185, 4651–4658. Hamada, S., Umemura, M., Shiono, T., Tanaka, K., Yahagi, A., Begum, M.D., Oshiro, K., Okamoto, Y., Watanabe, H., Kawakami, K., et al. (2008). IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J. Immunol. 181, 3456–3463. de Boer, O.J., van der Meer, J.J., Teeling, P., van der Loos, C.M., Idu, M.M., van Maldegem, F., Aten, J., and van der Wal, A.C. (2010). Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J. Pathol. 220, 499–508. Doreau, A., Belot, A., Bastid, J., Riche, B., Trescol-Biemont, M.C., Ranchin, B., Fabien, N., Cochat, P., Pouteil-Noble, C., Trolliet, P., et al. (2009). Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat. Immunol. 10, 778–785. Hinrichs, C.S., Kaiser, A., Paulos, C.M., Cassard, L., Sanchez-Perez, L., Heemskerk, B., Wrzesinski, C., Borman, Z.A., Muranski, P., and Restifo, N.P. (2009). Type 17 CD8+ T cells display enhanced antitumor immunity. Blood 114, 596–599. Hirahara, K., Ghoreschi, K., Laurence, A., Yang, X.P., Kanno, Y., and O’Shea, J.J. (2010). Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 21, 425–434. Emamaullee, J.A., Davis, J., Merani, S., Toso, C., Elliott, J.F., Thiesen, A., and Shapiro, A.M. (2009). Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 58, 1302–1311. Hirota, K., Hashimoto, M., Yoshitomi, H., Tanaka, S., Nomura, T., Yamaguchi, T., Iwakura, Y., Sakaguchi, N., and Sakaguchi, S. (2007). T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204, 41–47. Fallon, P.G., Ballantyne, S.J., Mangan, N.E., Barlow, J.L., Dasvarma, A., Hewett, D.R., McIlgorm, A., Jolin, H.E., and McKenzie, A.N. (2006). Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116. Ho, A.W., Shen, F., Conti, H.R., Patel, N., Childs, E.E., Peterson, A.C., Hernández-Santos, N., Kolls, J.K., Kane, L.P., Ouyang, W., and Gaffen, S.L. (2010). IL-17RC is required for immune signaling via an extended SEF/IL17R signaling domain in the cytoplasmic tail. J. Immunol. 185, 1063–1070. Flierl, M.A., Rittirsch, D., Gao, H., Hoesel, L.M., Nadeau, B.A., Day, D.E., Zetoune, F.S., Sarma, J.V., Huber-Lang, M.S., Ferrara, J.L., and Ward, P.A. (2008). Adverse functions of IL-17A in experimental sepsis. FASEB J. 22, 2198–2205. Holland, D.B., Bojar, R.A., Farrar, M.D., and Holland, K.T. (2009). Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol. Lett. 290, 149–155. Freitas, A., Alves-Filho, J.C., Victoni, T., Secher, T., Lemos, H.P., Sônego, F., Cunha, F.Q., and Ryffel, B. (2009). IL-17 receptor signaling is required to control polymicrobial sepsis. J. Immunol. 182, 7846–7854. Hu, Y., Ota, N., Peng, I., Refino, C.J., Danilenko, D.M., Caplazi, P., and Ouyang, W. (2010). IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 184, 4307–4316. Gaboriau-Routhiau, V., Rakotobe, S., Lécuyer, E., Mulder, I., Lan, A., Bridonneau, C., Rochet, V., Pisi, A., De Paepe, M., Brandi, G., et al. (2009). The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689. Gaffen, S.L. (2009). Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9, 556–567. Geboes, L., Dumoutier, L., Kelchtermans, H., Schurgers, E., Mitera, T., Renauld, J.C., and Matthys, P. (2009). Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 60, 390–395. Genovese, M.C., Van den Bosch, F., Roberson, S.A., Bojin, S., Biagini, I.M., Ryan, P., and Sloan-Lancaster, J. (2010). LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-ofconcept study. Arthritis Rheum. 62, 929–939. Gerhardt, S., Abbott, W.M., Hargreaves, D., Pauptit, R.A., Davies, R.A., Needham, M.R., Langham, C., Barker, W., Aziz, A., Snow, M.J., et al. (2009). Structure of IL-17A in complex with a potent, fully human neutralizing antibody. J. Mol. Biol. 394, 905–921. Geyer, M., and Müller-Ladner, U. (2010). Actual status of antiinterleukin-1 therapies in rheumatic diseases. Curr. Opin. Rheumatol. 22, 246–251. Gomez-Rodriguez, J., Sahu, N., Handon, R., Davidson, T.S., Anderson, S.M., Kirby, M.R., August, A., and Schwartzberg, P.L. (2009). Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity 31, 587–597. Gorbacheva, V., Fan, R., Li, X., and Valujskikh, A. (2010). Interleukin-17 promotes early allograft inflammation. Am. J. Pathol. 177, 1265–1273. Gottlieb, A., Menter, A., Mendelsohn, A., Shen, Y.K., Li, S., Guzzo, C., Fretzin, S., Kunynetz, R., and Kavanaugh, A. (2009). Ustekinumab, a human interleukin Hueber, W., Patel, D.D., Dryja, T., Wright, A.M., Koroleva, I., Bruin, G., Antoni, C., Draelos, Z., Gold, M.H., Durez, P., et al. (2010). Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2, 52ra72. Hwang, S.Y., and Kim, H.Y. (2005). Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Mol. Cells 19, 180–184. Hymowitz, S.G., Filvaroff, E.H., Yin, J.P., Lee, J., Cai, L., Risser, P., Maruoka, M., Mao, W., Foster, J., Kelley, R.F., et al. (2001). IL-17s adopt a cystine knot fold: Structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 20, 5332–5341. Ishigame, H., Kakuta, S., Nagai, T., Kadoki, M., Nambu, A., Komiyama, Y., Fujikado, N., Tanahashi, Y., Akitsu, A., Kotaki, H., et al. (2009). Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119. Ito, R., Kita, M., Shin-Ya, M., Kishida, T., Urano, A., Takada, R., Sakagami, J., Imanishi, J., Iwakura, Y., Okanoue, T., et al. (2008). Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun. 377, 12–16. Ivanov, I.I., Atarashi, K., Manel, N., Brodie, E.L., Shima, T., Karaoz, U., Wei, D., Goldfarb, K.C., Santee, C.A., Lynch, S.V., et al. (2009). Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498. Iwakura, Y., Nakae, S., Saijo, S., and Ishigame, H. (2008). The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol. Rev. 226, 57–79. Izcue, A., Hue, S., Buonocore, S., Arancibia-Cárcamo, C.V., Ahern, P.P., Iwakura, Y., Maloy, K.J., and Powrie, F. (2008). Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity 28, 559–570. Immunity 34, February 25, 2011 ª2011 Elsevier Inc. 159 Immunity Review Jacobs, J.P., Wu, H.J., Benoist, C., and Mathis, D. (2009). IL-17-producing T cells can augment autoantibody-induced arthritis. Proc. Natl. Acad. Sci. USA 106, 21789–21794. Kappel, L.W., Goldberg, G.L., King, C.G., Suh, D.Y., Smith, O.M., Ligh, C., Holland, A.M., Grubin, J., Mark, N.M., Liu, C., et al. (2009). IL-17 contributes to CD4-mediated graft-versus-host disease. Blood 113, 945–952. Kebir, H., Ifergan, I., Alvarez, J.I., Bernard, M., Poirier, J., Arbour, N., Duquette, P., and Prat, A. (2009). Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 66, 390–402. Kimball, A.B., Gordon, K.B., Langley, R.G., Menter, A., Perdok, R.J., and Valdes, J.; ABT-874 Study Investigators. (2011). Efficacy and safety of ABT-874, a monoclonal anti-interleukin 12/23 antibody, for the treatment of chronic plaque psoriasis: 36-week observation/retreatment and 60-week open-label extension phases of a randomized phase II trial. J. Am. Acad. Dermatol. 64, 263–274. Kleinschek, M.A., Owyang, A.M., Joyce-Shaikh, B., Langrish, C.L., Chen, Y., Gorman, D.M., Blumenschein, W.M., McClanahan, T., Brombacher, F., Hurst, S.D., et al. (2007). IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 204, 161–170. Kleinschek, M.A., Boniface, K., Sadekova, S., Grein, J., Murphy, E.E., Turner, S.P., Raskin, L., Desai, B., Faubion, W.A., de Waal Malefyt, R., et al. (2009). Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 206, 525–534. Koga, C., Kabashima, K., Shiraishi, N., Kobayashi, M., and Tokura, Y. (2008). Possible pathogenic role of Th17 cells for atopic dermatitis. J. Invest. Dermatol. 128, 2625–2630. Kolls, J.K., and Lindén, A. (2004). Interleukin-17 family members and inflammation. Immunity 21, 467–476. Komiyama, Y., Nakae, S., Matsuki, T., Nambu, A., Ishigame, H., Kakuta, S., Sudo, K., and Iwakura, Y. (2006). IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177, 566–573. Korn, T., Bettelli, E., Oukka, M., and Kuchroo, V.K. (2009). IL-17 and Th17 Cells. Annu. Rev. Immunol. 27, 485–517. Kreymborg, K., Etzensperger, R., Dumoutier, L., Haak, S., Rebollo, A., Buch, T., Heppner, F.L., Renauld, J.C., and Becher, B. (2007). IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 179, 8098–8104. Kryczek, I., Wei, S., Szeliga, W., Vatan, L., and Zou, W. (2009). Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 114, 357–359. Kuestner, R.E., Taft, D.W., Haran, A., Brandt, C.S., Brender, T., Lum, K., Harder, B., Okada, S., Ostrander, C.D., Kreindler, J.L., et al. (2007). Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. 179, 5462–5473. Lajoie, S., Lewkowich, I.P., Suzuki, Y., Clark, J.R., Sproles, A.A., Dienger, K., Budelsky, A.L., and Wills-Karp, M. (2010). Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat. Immunol. 11, 928–935. Lee, J., Ho, W.H., Maruoka, M., Corpuz, R.T., Baldwin, D.T., Foster, J.S., Goddard, A.D., Yansura, D.G., Vandlen, R.L., Wood, W.I., and Gurney, A.L. (2001). IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J. Biol. Chem. 276, 1660–1664. Lee, Y.K., Turner, H., Maynard, C.L., Oliver, J.R., Chen, D., Elson, C.O., and Weaver, C.T. (2009). Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107. LeibundGut-Landmann, S., Gross, O., Robinson, M.J., Osorio, F., Slack, E.C., Tsoni, S.V., Schweighoffer, E., Tybulewicz, V., Brown, G.D., Ruland, J., and Reis e Sousa, C. (2007). Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8, 630–638. Leonardi, C.L., Kimball, A.B., Papp, K.A., Yeilding, N., Guzzo, C., Wang, Y., Li, S., Dooley, L.T., and Gordon, K.B.; PHOENIX 1 study investigators. (2008). Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–1674. 160 Immunity 34, February 25, 2011 ª2011 Elsevier Inc. Leppkes, M., Becker, C., Ivanov, I.I., Hirth, S., Wirtz, S., Neufert, C., Pouly, S., Murphy, A.J., Valenzuela, D.M., Yancopoulos, G.D., et al. (2009). RORgammaexpressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology 136, 257–267. Li, H., Chen, J., Huang, A., Stinson, J., Heldens, S., Foster, J., Dowd, P., Gurney, A.L., and Wood, W.I. (2000). Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc. Natl. Acad. Sci. USA 97, 773–778. Li, J., Simeoni, E., Fleury, S., Dudler, J., Fiorini, E., Kappenberger, L., von Segesser, L.K., and Vassalli, G. (2006). Gene transfer of soluble interleukin17 receptor prolongs cardiac allograft survival in a rat model. Eur. J. Cardiothorac. Surg. 29, 779–783. Liang, S.C., Long, A.J., Bennett, F., Whitters, M.J., Karim, R., Collins, M., Goldman, S.J., Dunussi-Joannopoulos, K., Williams, C.M., Wright, J.F., and Fouser, L.A. (2007). An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 179, 7791–7799. Lin, Y., Ritchea, S., Logar, A., Slight, S., Messmer, M., Rangel-Moreno, J., Guglani, L., Alcorn, J.F., Strawbridge, H., Park, S.M., et al. (2009). Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31, 799–810. Martin-Orozco, N., Muranski, P., Chung, Y., Yang, X.O., Yamazaki, T., Lu, S., Hwu, P., Restifo, N.P., Overwijk, W.W., and Dong, C. (2009). T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31, 787–798. McGeachy, M.J., and Cua, D.J. (2008). Th17 cell differentiation: The long and winding road. Immunity 28, 445–453. Mchenga, S.S., Wang, D., Li, C., Shan, F., and Lu, C. (2008). Inhibitory effect of recombinant IL-25 on the development of dextran sulfate sodium-induced experimental colitis in mice. Cell. Mol. Immunol. 5, 425–431. Milner, J.D., Sandler, N.G., and Douek, D.C. (2010). Th17 cells, Job’s syndrome and HIV: Opportunities for bacterial and fungal infections. Curr Opin HIV AIDS 5, 179–183. Mitsdoerffer, M., Lee, Y., Jäger, A., Kim, H.J., Korn, T., Kolls, J.K., Cantor, H., Bettelli, E., and Kuchroo, V.K. (2010). Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA 107, 14292–14297. Moro, K., Yamada, T., Tanabe, M., Takeuchi, T., Ikawa, T., Kawamoto, H., Furusawa, J., Ohtani, M., Fujii, H., and Koyasu, S. (2010). Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544. Moseley, T.A., Haudenschild, D.R., Rose, L., and Reddi, A.H. (2003). Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14, 155–174. Mosmann, T.R., Cherwinski, H., Bond, M.W., Giedlin, M.A., and Coffman, R.L. (1986). Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136, 2348–2357. Murphy, C.A., Langrish, C.L., Chen, Y., Blumenschein, W., McClanahan, T., Kastelein, R.A., Sedgwick, J.D., and Cua, D.J. (2003). Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957. Nair, R.P., Duffin, K.C., Helms, C., Ding, J., Stuart, P.E., Goldgar, D., Gudjonsson, J.E., Li, Y., Tejasvi, T., Feng, B.J., et al; Collaborative Association Study of Psoriasis. (2009). Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat. Genet. 41, 199–204. Nakae, S., Komiyama, Y., Nambu, A., Sudo, K., Iwase, M., Homma, I., Sekikawa, K., Asano, M., and Iwakura, Y. (2002). Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387. Nakae, S., Nambu, A., Sudo, K., and Iwakura, Y. (2003). Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171, 6173–6177. Nakajima, A., Matsuki, T., Komine, M., Asahina, A., Horai, R., Nakae, S., Ishigame, H., Kakuta, S., Saijo, S., and Iwakura, Y. (2010). TNF, but not IL-6 and IL-17, is crucial for the development of T cell-independent psoriasis-like dermatitis in Il1rn-/- mice. J. Immunol. 185, 1887–1893. Immunity Review Neill, D.R., Wong, S.H., Bellosi, A., Flynn, R.J., Daly, M., Langford, T.K., Bucks, C., Kane, C.M., Fallon, P.G., Pannell, R., et al. (2010). Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370. Saijo, S., Ikeda, S., Yamabe, K., Kakuta, S., Ishigame, H., Akitsu, A., Fujikado, N., Kusaka, T., Kubo, S., Chung, S.H., et al. (2010). Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691. Numasaki, M., Fukushi, J., Ono, M., Narula, S.K., Zavodny, P.J., Kudo, T., Robbins, P.D., Tahara, H., and Lotze, M.T. (2003). Interleukin-17 promotes angiogenesis and tumor growth. Blood 101, 2620–2627. Sato, K., Suematsu, A., Okamoto, K., Yamaguchi, A., Morishita, Y., Kadono, Y., Tanaka, S., Kodama, T., Akira, S., Iwakura, Y., et al. (2006). Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203, 2673–2682. O’Connor, W., Jr., Kamanaka, M., Booth, C.J., Town, T., Nakae, S., Iwakura, Y., Kolls, J.K., and Flavell, R.A. (2009). A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 10, 603–609. Ogawa, A., Andoh, A., Araki, Y., Bamba, T., and Fujiyama, Y. (2004). Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 110, 55–62. Ogura, H., Murakami, M., Okuyama, Y., Tsuruoka, M., Kitabayashi, C., Kanamoto, M., Nishihara, M., Iwakura, Y., and Hirano, T. (2008). Interleukin17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29, 628–636. Owyang, A.M., Zaph, C., Wilson, E.H., Guild, K.J., McClanahan, T., Miller, H.R., Cua, D.J., Goldschmidt, M., Hunter, C.A., Kastelein, R.A., and Artis, D. (2006). Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203, 843–849. Oyoshi, M.K., Murphy, G.F., and Geha, R.S. (2009). Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol 124, 485–493, 493 e481. Patel, A.M., and Moreland, L.W. (2010). Interleukin-6 inhibition for treatment of rheumatoid arthritis: A review of tocilizumab therapy. Drug Des Devel Ther 4, 263–278. Petermann, F., Rothhammer, V., Claussen, M.C., Haas, J.D., Blanco, L.R., Heink, S., Prinz, I., Hemmer, B., Kuchroo, V.K., Oukka, M., and Korn, T. (2010). gd T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity 33, 351–363. Pichavant, M., Goya, S., Meyer, E.H., Johnston, R.A., Kim, H.Y., Matangkasombut, P., Zhu, M., Iwakura, Y., Savage, P.B., DeKruyff, R.H., et al. (2008). Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J. Exp. Med. 205, 385–393. Price, A.E., Liang, H.E., Sullivan, B.M., Reinhardt, R.L., Eisley, C.J., Erle, D.J., and Locksley, R.M. (2010). Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 107, 11489–11494. Raffatellu, M., Santos, R.L., Verhoeven, D.E., George, M.D., Wilson, R.P., Winter, S.E., Godinez, I., Sankaran, S., Paixao, T.A., Gordon, M.A., et al. (2008). Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14, 421–428. Res, P.C., Piskin, G., de Boer, O.J., van der Loos, C.M., Teeling, P., Bos, J.D., and Teunissen, M.B. (2010). Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS ONE 5, e14108. Reynolds, J.M., Angkasekwinai, P., and Dong, C. (2010). IL-17 family member cytokines: Regulation and function in innate immunity. Cytokine Growth Factor Rev. 21, 413–423. Rizzo, H.L., Kagami, S., Phillips, K.G., Kurtz, S.E., Jacques, S.L., and Blauvelt, A. (2011). IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J. Immunol. 186, 1495–1502. Roark, C.L., French, J.D., Taylor, M.A., Bendele, A.M., Born, W.K., and O’Brien, R.L. (2007). Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J. Immunol. 179, 5576–5583. Saenz, S.A., Noti, M., and Artis, D. (2010a). Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 31, 407–413. Saenz, S.A., Siracusa, M.C., Perrigoue, J.G., Spencer, S.P., Urban, J.F., Jr., Tocker, J.E., Budelsky, A.L., Kleinschek, M.A., Kastelein, R.A., Kambayashi, T., et al. (2010b). IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature 464, 1362–1366. Schnyder-Candrian, S., Togbe, D., Couillin, I., Mercier, I., Brombacher, F., Quesniaux, V., Fossiez, F., Ryffel, B., and Schnyder, B. (2006). Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 203, 2715–2725. Segal, B.M., Constantinescu, C.S., Raychaudhuri, A., Kim, L., Fidelus-Gort, R., and Kasper, L.H.; Ustekinumab MS Investigators. (2008). Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: A phase II, double-blind, placebocontrolled, randomised, dose-ranging study. Lancet Neurol. 7, 796–804. Shi, Y., Ullrich, S.J., Zhang, J., Connolly, K., Grzegorzewski, K.J., Barber, M.C., Wang, W., Wathen, K., Hodge, V., Fisher, C.L., et al. (2000). A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J. Biol. Chem. 275, 19167–19176. Shibata, K., Yamada, H., Hara, H., Kishihara, K., and Yoshikai, Y. (2007). Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178, 4466– 4472. Shichita, T., Sugiyama, Y., Ooboshi, H., Sugimori, H., Nakagawa, R., Takada, I., Iwaki, T., Okada, Y., Iida, M., Cua, D.J., et al. (2009). Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950. Sonnenberg, G.F., Monticelli, L.A., Elloso, M.M., Fouser, L.A., and Artis, D. (2011). CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34, 122–134. Stamp, L.K., Easson, A., Lehnigk, U., Highton, J., and Hessian, P.A. (2008). Different T cell subsets in the nodule and synovial membrane: Absence of interleukin-17A in rheumatoid nodules. Arthritis Rheum. 58, 1601–1608. Starnes, T., Broxmeyer, H.E., Robertson, M.J., and Hromas, R. (2002). Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J. Immunol. 169, 642–646. Swaidani, S., Bulek, K., Kang, Z., Liu, C., Lu, Y., Yin, W., Aronica, M., and Li, X. (2009). The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J. Immunol. 182, 1631–1640. Takahashi, N., Vanlaere, I., de Rycke, R., Cauwels, A., Joosten, L.A., Lubberts, E., van den Berg, W.B., and Libert, C. (2008). IL-17 produced by Paneth cells drives TNF-induced shock. J. Exp. Med. 205, 1755–1761. Tamachi, T., Maezawa, Y., Ikeda, K., Kagami, S., Hatano, M., Seto, Y., Suto, A., Suzuki, K., Watanabe, N., Saito, Y., et al. (2006). IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J. Allergy Clin. Immunol. 118, 606–614. Tartour, E., Fossiez, F., Joyeux, I., Galinha, A., Gey, A., Claret, E., SastreGarau, X., Couturier, J., Mosseri, V., Vives, V., et al. (1999). Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 59, 3698–3704. Terashima, A., Watarai, H., Inoue, S., Sekine, E., Nakagawa, R., Hase, K., Iwamura, C., Nakajima, H., Nakayama, T., and Taniguchi, M. (2008). A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J. Exp. Med. 205, 2727–2733. Van Maele, L., Carnoy, C., Cayet, D., Songhet, P., Dumoutier, L., Ferrero, I., Janot, L., Erard, F., Bertout, J., Leger, H., et al. (2010). TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J. Immunol. 185, 1177–1185. Venken, K., Hellings, N., Hensen, K., Rummens, J.L., and Stinissen, P. (2010). Memory CD4+CD127high T cells from patients with multiple sclerosis produce IL-17 in response to myelin antigens. J. Neuroimmunol. 226, 185–191. Vokaer, B., Van Rompaey, N., Lemaı̂tre, P.H., Lhommé, F., Kubjak, C., Benghiat, F.S., Iwakura, Y., Petein, M., Field, K.A., Goldman, M., et al. Immunity 34, February 25, 2011 ª2011 Elsevier Inc. 161 Immunity Review (2010). Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. J. Immunol. 185, 3417–3425. Wang, L., Yi, T., Kortylewski, M., Pardoll, D.M., Zeng, D., and Yu, H. (2009). IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med. 206, 1457–1464. Wang, Q., Li, H., Yao, Y., Xia, D., and Zhou, J. (2010a). The overexpression of heparin-binding epidermal growth factor is responsible for Th17-induced airway remodeling in an experimental asthma model. J. Immunol. 185, 834–841. Wang, Y.H., Voo, K.S., Liu, B., Chen, C.Y., Uygungil, B., Spoede, W., Bernstein, J.A., Huston, D.P., and Liu, Y.J. (2010b). A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J. Exp. Med. 207, 2479–2491. Weaver, C.T., Hatton, R.D., Mangan, P.R., and Harrington, L.E. (2007). IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852. Wilson, N.J., Boniface, K., Chan, J.R., McKenzie, B.S., Blumenschein, W.M., Mattson, J.D., Basham, B., Smith, K., Chen, T., Morel, F., et al. (2007). Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8, 950–957. Wilson, M.S., Madala, S.K., Ramalingam, T.R., Gochuico, B.R., Rosas, I.O., Cheever, A.W., and Wynn, T.A. (2010). Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 207, 535–552. Wu, Q., Martin, R.J., Rino, J.G., Breed, R., Torres, R.M., and Chu, H.W. (2007). IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 9, 78–86. Wu, S., Rhee, K.J., Albesiano, E., Rabizadeh, S., Wu, X., Yen, H.R., Huso, D.L., Brancati, F.L., Wick, E., McAllister, F., et al. (2009). A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15, 1016–1022. Wu, H.J., Ivanov, I.I., Darce, J., Hattori, K., Shima, T., Umesaki, Y., Littman, D.R., Benoist, C., and Mathis, D. (2010). Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827. Yamaguchi, Y., Fujio, K., Shoda, H., Okamoto, A., Tsuno, N.H., Takahashi, K., and Yamamoto, K. (2007). IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J. Immunol. 179, 7128–7136. 162 Immunity 34, February 25, 2011 ª2011 Elsevier Inc. Yang, X.O., Chang, S.H., Park, H., Nurieva, R., Shah, B., Acero, L., Wang, Y.H., Schluns, K.S., Broaddus, R.R., Zhu, Z., and Dong, C. (2008a). Regulation of inflammatory responses by IL-17F. J. Exp. Med. 205, 1063–1075. Yang, X.O., Pappu, B.P., Nurieva, R., Akimzhanov, A., Kang, H.S., Chung, Y., Ma, L., Shah, B., Panopoulos, A.D., Schluns, K.S., et al. (2008b). T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 28, 29–39. Yi, T., Zhao, D., Lin, C.L., Zhang, C., Chen, Y., Todorov, I., LeBon, T., Kandeel, F., Forman, S., and Zeng, D. (2008). Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood 112, 2101–2110. Yoshitomi, H., Sakaguchi, N., Kobayashi, K., Brown, G.D., Tagami, T., Sakihama, T., Hirota, K., Tanaka, S., Nomura, T., Miki, I., et al. (2005). A role for fungal beta-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201, 949–960. Zaph, C., Du, Y., Saenz, S.A., Nair, M.G., Perrigoue, J.G., Taylor, B.C., Troy, A.E., Kobuley, D.E., Kastelein, R.A., Cua, D.J., et al. (2008). Commensaldependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 205, 2191–2198. Zenewicz, L.A., Yancopoulos, G.D., Valenzuela, D.M., Murphy, A.J., Stevens, S., and Flavell, R.A. (2008). Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29, 947–957. Zhao, A., Urban, J.F., Jr., Sun, R., Stiltz, J., Morimoto, M., Notari, L., Madden, K.B., Yang, Z., Grinchuk, V., Ramalingam, T.R., et al. (2010). Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J. Immunol. 185, 6921–6929. Zheng, Y., Valdez, P.A., Danilenko, D.M., Hu, Y., Sa, S.M., Gong, Q., Abbas, A.R., Modrusan, Z., Ghilardi, N., de Sauvage, F.J., and Ouyang, W. (2008). Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289. Zhou, L., and Littman, D.R. (2009). Transcriptional regulatory networks in Th17 cell differentiation. Curr. Opin. Immunol. 21, 146–152. Zhu, S., Pan, W., Shi, P., Gao, H., Zhao, F., Song, X., Liu, Y., Zhao, L., Li, X., Shi, Y., and Qian, Y. (2010). Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J. Exp. Med. 207, 2647–2662. Zou, W., and Restifo, N.P. (2010). T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 10, 248–256.