Test 1
advertisement

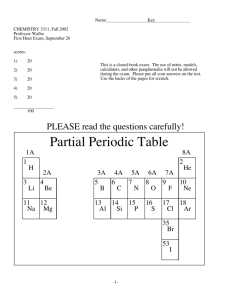
1 Test 1 - Chem 145 Sp 04 Dr. Duffy-Matzner Name ________________________ Lab - A1 or A2 or A3 Part I - Multiple Choice - place the letter for best answer in the blank (2 pts each). ____ 1. The total number of valence electrons available for CHCl3 would be: a) 32 b) 26 c) 24 d) 4 ____ 2. Which of the following compounds would NOT have a tetrahedral electronic geometry: a) CCl4 b) H2O c) NH3 d) CO ____ 3. Which of the following could have hydrogen bonding? a) CH4 b) H2S c) CH3OH d) CH3OCH3 ____ 4. This reaction will shift to the right if: CH4 (g) + 2 O2 a) H2O is added. b) O2 is removed. 2 H2O (l) + 2 CO2 (g) c) CO2 is removed. d) CH4 is removed. ____ 5. Which of the following is false concerning the equilibrium constant: a) It is a constant regardless of the quantities for the reagents. b) It will change if the temperature is changed. c) A large constant means that the formation of products is favored over reactants. d) A large constant means that the reaction will have a fast rate. ____ 6. In the reaction ,A B, 10 grams of B were expected, but the % yield was 70%. How much of B was actually isolated? a) .70 grams b) 3.0 grams ____ 7. Find the conjugate base for HBrO in: b) BrO-1 (aq) a)HBrO (aq) c) 7.0 grams d) 13 grams H3O+(aq) + BrO–1(aq) HBrO (aq) + H2O (l) d) OH-1 (aq) c) H2O (l) ____ 8. Which of the following particles would have the highest RBE? a) alpha b) positrons c) gamma d) beta ____ 9. Chose the reaction that represents a positron emission. 14 a) 14 C 6 5 238 c) U 92 0 B n 0 14 e b) 1 1 + + U 92 0 + 6 239 C e 93 7 239 Np 0 N 1 239 d) 14 Pu 94 γ 0 0 + + e -1 2 ____ 10. An unknown could be pentane or 1-pentene or 1-cyclopentene. A few drops of a KMnO4 solution is added and the unknown solution turns purple. The unknown is _____. a) pentane b) 1-pentene c) cyclopentene d) can't tell ____ 11. The bond angles that we would expect to see in an alkene functional group are close to: a) 90 b) 109.5 c) 120 d)180 ____ 12. Which of the following is false for the molecular formula C5H10? a) It may contain a double bond. b) It must be a linear alkane. c) It may contain a ring. d) It would be named with a pent prefix. ____ 13. Which of the following is true for a hydrogenation reaction: a) Hydrogen is added across a sigma bond. b) Only alkanes will react in this reaction. c) The presence of metal catalyst is required for the reaction to occur. d) It is an acid/base reaction. ____ 14. Addition polymerization of CH2 = CHCl would give which of the following? a) H H C C H Cl b) n H H C Cl H C c) n H H C C Cl H H n Part 2 – Nomenclature and Structures (16 pts) 1. Give the IUPAC (International Union of Pure and Applied Chemistry) name or give the structure of the following: a) b) cis-1-tert-butyl-3-methylcyclopentane c) d) 4-propyl-3,3-dimethyl-1-cyclohexene Cl 3 (6 pts) 2. Draw the Lewis structure for PH3. What is the electronic and molecular geometry around the P? What type of intermolecular forces would be present? (6 pts) 3. Please answer the following questions for the trans isomer of CH3CH = CHCH(CH3)2: a) Draw the skeletal (shorthand) structure for this compound. b) Draw a geometric isomer of the original compound. c) Draw a constitutional (structural) isomer. Part 3 – Reactions – Fill in the blank area. Write no reaction if appropriate. (16 total points). a) Br2 light assume only monobromination products are formed b) H2O c) acid catalyst KMnO4 d) e) not cold H2SO4 4 Part 4 – Please show all of your work or give full explanations for your answers. (7 pts) 1. Please finish the following reaction (balance) and indicate the type of reaction. Then write the net ionic equation. Na2SO4 (aq) + Ba(ClO3)2 (aq) (5 pts) 2. If the half-life of N-16 is 7.2 seconds, what mass of an 84 mg sample will remain after 1.1 mintues? (8 pts) 3. Answer the following questions about the active ingrediant in THC. B OH A O C a) Determine the hybridizations of atoms: A __________ B __________ b) Determine the bond angles centered around: A __________ B __________ c) Determine the type of carbon (1° or 2° or 3° or 4°) for A __________ C __________ d) Circle the different types of functional groups that are present in this molecule. alkene aromatic alkyne alcohol ether ester amine amide (8 pts) 4. The addition of HBr to 1-pentene gives one major and one minor product, 2-bromopentane and 1-bromopentane, respectively. The addition of HBr to 2-pentene gives two products, 2-bromopentane and 3-bromopentane, in equal amounts. Write the reactions. Then explain the difference between the reactions of 1-pentene and 2-pentene with HBr.