2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr
advertisement

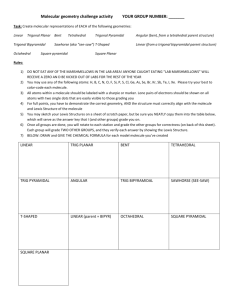
2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, electron geometry (eg) – Mother structure of VSEPR molecular geometry (mg) – Daughter Structure Ch9 1) Which of the following represent the Lewis structure for N? A) B) C) D) E) Answer: A 2) Which of the following represent the Lewis structure for Cl? A) B) C) D) E) Answer: B 3) Which of the following represent the Lewis structure for Mg? A) B) C) D) E) Answer: C 4) Which of the following represent the Lewis structure for Br⁻? A) B) C) D) E) Answer: D 5) Which of the following represent the Lewis structure for Ca2⁺? No valence electrons A) B) C) D) E) 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, Answer: E 6) Give the complete electronic configuration for Br-. A) 1s22s22p63s23p64s24p6 B) 1s22s22p63s23p64s24d104p6 C) 1s22s22p63s23p64s23d104p6 D) 1s22s22p63s23p64s23d104p5 E) 1s22s2p63s2p64s23d104p6 Answer: C 7) Give the complete electronic configuration for Ca2+. A) 1s22s22p63s24p6 B) 1s22s22p63s23p6 C) 1s22s22p63s23p5 D) 1s22s23p64s25p6 E) 1s22s2p63s2p6 Answer: B 8) Give the complete electronic configuration for S2-. A) 1s22s22p63s24p6 B) 1s22s22p63s23p6 C) 1s22s22p63s23p5 D) 1s22s23p64s25p6 E) 1s22s2p63s2p6 Answer: B 9) Place the following in order of decreasing magnitude of lattice energy. NaF RbBr KCl A) RbBr > NaF > KCl B) NaF > KCl > RbBr C) KCl > NaF > RbBr D) NaF > RbBr > KCl E) RbBr > KCl > NaF Answer: B 10) Place the following in order of increasing magnitude of lattice energy. CaO MgO A) MgO < CaO < SrS B) SrS < MgO < CaO C) SrS < CaO < MgO D) CaO < MgO < SrS E) CaO < SrS < MgO Answer: C SrS 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, 11) Identify the shortest bond. A) single covalent bond B) double covalent bond C) triple covalent bond D) all of the above bonds are the same length Answer: C 12) Identify the weakest bond. A) single covalent bond B) double covalent bond C) triple covalent bond D) all of the above bonds are the same strength Answer: A 13) Identify the strongest bond. A) single covalent bond B) double covalent bond C) triple covalent bond D) all of the above bonds are the same strength Answer: C 14) Identify the number of bonding pairs and lone pairs of electrons in water. A) 1 bonding pair and 1 lone pair B) 1 bonding pair and 2 lone pairs C) 2 bonding pairs and 2 lone pairs D) 2 bonding pairs and 1 lone pair E) 3 bonding pairs and 2 lone pairs Answer: C 15) Identify the compound with the largest dipole moment in the gas phase.( calculate the electron negativity difference) A) Cl2 B) ClF C) HF D) LiF Answer: D 16) Identify the compound with the smallest dipole moment in the gas phase. A) Cl2 B) ClF C) HF D) LiF Answer: A 2014Spring CHEM101 Ch9-10 Review Worksheet 17) Choose the bond below that is most polar. A) H-I B) H-Br C) H-F D) H-Cl E) C-H Answer: C 18) Choose the bond below that is least polar. A) P-F B) C-Br C) C-F D) C-I E) C-Cl Answer: D 19) Choose the best Lewis structure for BeF2. A) B) C) D) E) Answer: D 20) Choose the best Lewis structure for OCl2. A) B) C) D) E) Answer: E 21) Choose the best Lewis structure for CH2Cl2. A) B) C) D) Modified by Dr. Cheng-Yu Lai, 2014Spring CHEM101 Ch9-10 Review Worksheet E) Answer: B 22) Choose the best Lewis structure for XeI2. A) B) C) D) E) Answer: C 23) Give the number of valence electrons for ICl5. A) 36 B) 40 C) 42 D) 44 E) 46 Answer: C 24) Choose the best Lewis structure for ICl5. A) B) C) D) E) Modified by Dr. Cheng-Yu Lai, 2014Spring CHEM101 Ch9-10 Review Worksheet Answer: D 25) Choose the best Lewis structure for SF4. A) B) C) D) E) Answer: E 26) Choose the best Lewis structure for NO3⁻. A) B) C) D) E) Modified by Dr. Cheng-Yu Lai, 2014Spring CHEM101 Ch9-10 Review Worksheet Answer: A 27) Choose the best Lewis structure for SO42⁻. A) B) C) D) E) Answer: A 28) Choose the best Lewis structure for PO43⁻. A) B) Modified by Dr. Cheng-Yu Lai, 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, C) D) E) Answer: C 29) Draw the Lewis structure for CO32- including any valid resonance structures. Which of the following statements is TRUE? A) The CO32- ion contains one C—O single bond and two C=O double bonds. B) The CO32- ion contains two C—O single bonds and one C=O double bond. C) The CO32- ion contains three C—O double bonds. D) The CO32- ion contains two C—O single bonds and one C≡O triple bond. E) None of the above are true. Answer: B 30) Using Lewis structures and formal charge, which of the following ions is most stable? OCN⁻ ONC⁻ NOC⁻ A) OCN⁻ B) ONC⁻ C) NOC⁻ D) None of these ions are stable according to Lewis theory. E) All of these compounds are equally stable according to Lewis theory. Answer: A 31) Rank the following molecules in decreasing bond energy. Cl2 Br2 F2 A) I2 > Br2 > Cl2 > F2 B) Cl2 > Br2 > F2 > I2 C) I2 > Cl2 > Br2 > F2 D) Cl2 > I2 > F2 > Br2 Answer: B I2 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, 32) Use the bond energies provided to estimate ΔH°rxn for the reaction below. Bond Cl-Cl P-Cl PCl3(g) + Cl2(g) → PCl5(l) ΔH°rxn = ? Bond Energy (kJ/mol) 243 331 A) -243 kJ B) -419 kJ C) -662 kJ D) -67 kJ E) -905 kJ Answer: B 33) Use the bond energies provided to estimate ΔH°rxn for the reaction below. Bond C-H C-O C=O O=O O-H CH3OH(l) + 2 O2(g) → CO2(g) + 2 H2O(g) Bond Energy (kJ/mol) 414 360 799 498 464 ΔH°rxn = ? A) +473 kJ B) -91 kJ C) -486 kJ D) -392 kJ E) +206 kJ Answer: D 34) Use the bond energies provided to estimate ΔH°rxn for the reaction below. Bond Xe-F F-F XeF2 + 2 F2 → XeF6 Bond Energy (kJ/mol) 147 159 ΔH°rxn = ? A) -429 kJ B) +159 kJ C) -660 kJ D) +176 kJ E) -270 kJ Answer: E Ch10 35) Give the approximate bond angle for a molecule with an octahedral shape. A) 109.5° B) 180° 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, C) 120° D) 105° E) 90° Answer: E 36) How many of the following molecules are polar? BrCl3 CS2 SiF4 SO3 A) 1 B) 2 C) 3 D) 4 E) 0 Answer: A 37) How many of the following molecules are polar? PCl5 COS XeO3 SeBr2 A) 2 B) 0 C) 1 D) 3 E) 4 Answer: D electron geometry (eg) – VSPER Mother structure geometry 38) Give the electron geometry (eg), molecular geometry (mg), and hybridization for NH3. A) eg=tetrahedral, mg=trigonal planar, sp2 B) eg=trigonal pyramidal, mg=trigonal pyramidal, sp3 C) eg=tetrahedral, mg=trigonal pyramidal, sp3 D) eg=trigonal pyramidal, mg=tetrahedral, sp3 E) eg=trigonal planar, mg=trigonal planar, sp2 Answer: C 39) Give the electron geometry (eg), molecular geometry (mg), and hybridization for H2O. A) eg=tetrahedral, mg=bent, sp3 B) eg=trigonal pyramidal, mg=trigonal pyramidal, sp3 C) eg=tetrahedral, mg=trigonal pyramidal, sp3 D) eg=bent, mg=bent, sp2 E) eg=trigonal planar, mg=trigonal planar, sp2 Answer: A 40) Give the electron geometry (eg), molecular geometry (mg), and hybridization for XeF4. A) eg=tetrahedral, mg=tetrahedral, sp3 B) eg=trigonal pyramidal, mg=trigonal pyramidal, sp3 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, C) eg=octahedral, mg=square planar, sp3d2 D) eg=octahedral, mg=octahedral, sp3d2 E) eg=trigonal bipyramidal, mg=seesaw, sp3d Answer: C 41) Give the approximate bond angle for a molecule with an octahedral shape. A) 109.5° B) 180° C) 120° D) 105° E) 90° Answer: E 42) Determine the electron geometry (eg) and molecular geometry (mg) of CO32⁻. A) eg=tetrahedral, mg=tetrahedral B) eg=tetrahedral, mg=trigonal pyramidal C) eg=trigonal planar, mg=bent D) eg=trigonal planar, mg=trigonal planar E) eg=tetrahedral, mg=trigonal planar Answer: D 43) Determine the electron geometry (eg) and molecular geometry (mg) of SiF4. A) eg=tetrahedral, mg=trigonal pyramidal B) eg=octahedral, mg=square planar C) eg=trigonal bipyramidal, mg=trigonal pyramidal D) eg=tetrahedral, mg=bent E) eg=tetrahedral, mg=tetrahedral Answer: E 44) Determine the electron geometry (eg) and molecular geometry (mg) of PF5. A) eg=trigonal bipyramidal, mg=trigonal bipyramidal B) eg=octahedral, mg=octahedral C) eg=trigonal bipyramidal, mg=tetrahedral D) eg=tetrahedral, mg=trigonal pyramidal E) eg=trigonal planar, mg=octahedral Answer: A 45) Determine the electron geometry (eg) and molecular geometry (mg) of CO2. A) eg=tetrahedral, mg=tetrahedral B) eg=linear, mg=trigonal planar C) eg=trigonal planar, mg=bent D) eg=linear, mg=linear E) eg=trigonal planar, mg=trigonal planar Answer: D 46) Determine the electron geometry (eg) and molecular geometry (mg) of ICl3. A) eg=trigonal planar, mg=trigonal planar B) eg=trigonal bipyramidal, mg= T-shape C) eg=trigonal planar, mg=bent D) eg=trigonal bipyramidal, mg=see-saw 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, E) eg=tetrahedral, mg=trigonal pyramidal Answer: B 47) Determine the electron geometry (eg) and molecular geometry (mg) of XeF2. A) eg=trigonal bipyramidal, mg=bent B) eg=linear, mg=linear C) eg=tetrahedral, mg=linear D) eg=trigonal bipyramidal, mg=linear E) eg=tetrahedral, mg=bent Answer: D 48) Determine the electron geometry (eg) and molecular geometry (mg) of XeF4. A) eg=tetrahedral, mg=tetrahedral B) eg=linear, eg=linear C) eg=tetrahedral, mg=bent D) eg=trigonal bipyramidal, mg=tetrahedral E) eg=octahedral, mg=square planar Answer: E 49) Consider the molecule below. Determine the molecular geometry at each of the 2 labeled carbons. A) C1 = tetrahedral, C2 = linear B) C1 = trigonal planar, C2= bent C) C1 = bent, C2 = trigonal planar D) C1 = trigonal planar, C2 = tetrahedral E) C1 = trigonal pyramidal, C2 = see-saw Answer: D 50) Place the following in order of increasing F-A-F bond angle, where A represents the central atom in each molecule. PF3 OF2 PF4⁺ A) PF3 < OF2 < PF4⁺ B) OF2 < PF3 < PF4⁺ C) OF2 < PF4⁺ < PF3 D) PF4⁺ < OF2 < PF3 E) PF4⁺ < PF3 < OF2 Answer: B 51) Place the following in order of decreasing X-A-X bond angle, where A represents the central atom and X represents the outer atoms in each molecule. 2014Spring CHEM101 Ch9-10 Review Worksheet N2O NCl3 Modified by Dr. Cheng-Yu Lai, NO2⁻ A) NCl3 > NO2⁻ > N2O B) NO2⁻ > N2O > NCl3 C) N2O > NO2⁻ > NCl3 D) NCl3 > N2O > NO2⁻ E) N2O > NCl3 > NO2⁻ Answer: C 52) How many of the following molecules are polar? BrCl3 CS2 SiF4 SO3 A) 1 B) 2 C) 3 D) 4 E) 0 Answer: A 53) Determine the electron geometry (eg), molecular geometry (mg), and polarity of PCl3. A) eg=tetrahedral, mg=bent, polar B) eg=trigonal planar, mg=trigonal planar, nonpolar C) eg=linear, mg=linear, nonpolar D) eg=tetrahedral, mg=trigonal pyramidal, polar E) eg=trigonal pyramidal, mg=trigonal pyramidal, polar Answer: D 54) Determine the electron geometry, molecular geometry and polarity of SF6. A) eg=trigonal bipyramidal, mg=trigonal bipyramidal, nonpolar B) eg=tetrahedral, mg=tetrahedral, polar C) eg=trigonal bipyramidal, mg=see-saw, polar D) eg=octahedral, mg=trigonal bipyramidal, nonpolar E) eg=octahedral, mg=octahedral, nonpolar Answer: E 55) Determine the electron geometry (eg), molecular geometry(mg) and polarity of XeO3. A) eg=trigonal planar, mg=trigonal planar, nonpolar B) eg=tetrahedral, mg=trigonal pyramidal, polar C) eg=trigonal planar, mg=trigonal pyramidal, polar D) eg=trigonal bipyramidal, mg=trigonal planar, nonpolar E) eg=octahedral, mg=tetrahedral, nonpolar Answer: B 56) Place the following in order of increasing dipole moment. I. BCl3 A) I < II = III II. BIF2 III. BClF2 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, B) II < III < I C) I < II < III D) II < I < III E) I < III < II Answer: E 57) Place the following in order of decreasing dipole moment. I. cis-CHCl=CHCl II. trans-CHCl=CHCI III. cis-CHF=CHF A) III > I > II B) II > I > III C) I > III > II D) II > III > I E) I = III > II Answer: A 58) Give the electron geometry (eg), molecular geometry (mg), and hybridization for NH3. A) eg=tetrahedral, mg=trigonal planar, sp2 B) eg=trigonal pyramidal, mg=trigonal pyramidal, sp3 C) eg=tetrahedral, mg=trigonal pyramidal, sp3 D) eg=trigonal pyramidal, mg=tetrahedral, sp3 E) eg=trigonal planar, mg=trigonal planar, sp2 Answer: C 59) Give the electron geometry (eg), molecular geometry (mg), and hybridization for H2O. A) eg=tetrahedral, mg=bent, sp3 B) eg=trigonal pyramidal, mg=trigonal pyramidal, sp3 C) eg=tetrahedral, mg=trigonal pyramidal, sp3 D) eg=bent, mg=bent, sp2 E) eg=trigonal planar, mg=trigonal planar, sp2 Answer: A 60) Give the electron geometry (eg), molecular geometry (mg), and hybridization for XeF4. A) eg=tetrahedral, mg=tetrahedral, sp3 B) eg=trigonal pyramidal, mg=trigonal pyramidal, sp3 C) eg=octahedral, mg=square planar, sp3d2 D) eg=octahedral, mg=octahedral, sp3d2 E) eg=trigonal bipyramidal, mg=seesaw, sp3d Answer: C 61) Give the hybridization for the O in OF2. A) sp B) sp3 C) sp2 D) sp3d E) sp3d2 Answer: B 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, 62) Give the hybridization for the S in SO3. A) sp B) sp3 C) sp2 D) sp3d E) sp3d2 Answer: C 63) Give the hybridization for the Br in BrO4⁻. A) sp B) sp3d2 C) sp3d D) sp3 E) sp2 Answer: D 64) Give the hybridization for the S in SF6. A) sp B) sp2 C) sp3 D) sp3d E) sp3d2 Answer: E 65) Give the hybridization for the Br in BrF5. A) sp3d2 B) sp3d C) sp3 D) sp2 E) sp Answer: A 66) Give the hybridization for the Br in BrCl3. A) sp3d2 B) sp3d C) sp3 D) sp2 E) sp Answer: B 66) How many of the following molecules have sp2 hybridization on the central atom? HCN A) 4 B) 3 SO2 OCl2 XeCl2 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, C) 2 D) 1 E) 0 Answer: D 67) Use the molecular orbital diagram shown to determine which of the following is most stable. A) F2 B) F22⁺ C) Ne22⁺ D) O22⁺ E) F22⁻ Answer: D 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, 68) Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A) O22⁻ B) Ne22⁺ C) O22⁺ D) F22⁺ E) None of the above are paramagnetic. Answer: D 2014Spring CHEM101 Ch9-10 Review Worksheet Modified by Dr. Cheng-Yu Lai, 69) Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A) B22⁺ B) B22⁻ C) N22⁺ D) C22⁻ E) B2 Answer: E