CONTROL OF BREATHING
advertisement

Post-Baccalaureate
Jason H. Mateika Ph.D.
CONTROL OF BREATHING
Outline:
Role of brainstem and pons in establishing respiratory rhythm
Medullary Respiratory Centers (recent findings)
Patterns of Respiratory Neuron Activity
Function of Respiratory Neurons
Origin of the respiratory rhythm and developmental changes
Muscles of respiration
Neuronal and respiratory muscle activity during inspiration and expiration:
Upper airway structures
1
Post-Baccalaureate
Jason H. Mateika Ph.D.
Control of Breathing - Overview: Breathing occurs rhythmically. This rhythmicity is generated within
respiratory centers that are located in the medulla and pons. Many respiratory neurons located in the
medulla have axons that travel down to the spinal cord and synapse onto interneurons or motor neurons
located at the cervical, thoracic or lumbar regions of the spinal cord. The spinal neurons that receive
input from the medullary neurons form nerves that exit the spinal cord and innervate the muscles of
inspiration and expiration. Once the inspiratory muscles contract a negative pressure is generated which
causes air to travel from the atmosphere to our lungs. The depth and frequency of breathing is important
because these breathing components help to maintain homeostatic levels of oxygen, carbon dioxide and
hydrogen ions in arterial blood. A number of receptors that sense changes in lung volume or arterial
levels of oxygen, carbon dioxide or hydrogen ion concentration feedback to the medullary respiratory
neurons which influence the depth and frequency of breathing. In addition, temperature and pain
influence breathing via other centers (reticular formation) that feedback to the respiratory centers.
Additionally, the level of arousal (e.g. wake versus sleep) and emotions will influence breathing. Lastly,
breathing is also under voluntary control from the cerebral cortex (i.e. speaking, breath holding). The
voluntary pathways that control breathing bypass the respiratory centers in the medulla and directly
affect the respiratory motor neurons that are located in the spinal cord.
2
Post-Baccalaureate
Jason H. Mateika Ph.D.
Control of Breathing - Role of brainstem and pons in establishing respiratory rhythm:
Historical Perspective: Investigators believed initially that control centers located in the medulla and pons
generated the rhythmic pattern of breathing. The medullary respiratory center was considered the primary
respiratory control center consisting of two neuronal clusters the dorsal respiratory group (DRG) or nucleus
tractus solitarius (NTS) and the ventral respiratory group (VRG). Additionally, investigators believed that
the pontine respiratory center influenced the output of the medullary respiratory center. The pontine
respiratory center was thought to consist of the pneumotaxic center and the apneustic center. Investigators
believed that impulses from the pneumotaxic center inhibited inspiratory neurons in the DRG, while
impulses from the apneustic center prolonged the activity of the inspiratory neurons.
To determine the potential role of the pons and the medulla in the control of breathing, Lumsden completed
a series of transection experiments. He showed with the vagi intact that removal of higher brain structures,
such as the cerebral cortex, (transection A – upper right diagram) did not influence the basic respiratory
rhythm. However, a transection made in the upper 1/3 of the pons (transection B – upper right diagram)
resulted in a slower breathing rate and an increase in tidal volume. A transection made on the border of the
pons and medulla (i.e. eliminating inputs from the pneumotaxic & apneustic center) produced a variable
breathing pattern (gasping). These results suggested that inspiration is critically dependent on the lower
portion of the pons (apneustic center). Additionally, input from the upper portion of the pons may be
necessary to inhibit inspiration.
3
Post-Baccalaureate
Jason H. Mateika Ph.D.
Control of Breathing - Medullary Respiratory Centers (recent findings): No specific groups of neurons
have been found in the region that was historically referred to as the apneustic center. Thus, theories
regarding this center have been abandoned. The pneumotaxic center is now referred to as the pontine
respiratory group (PRG) and it is comprised of expiratory neurons in the medial parabrachial nucleus and
inspiratory neurons in the lateral parabrachial nucleus and Kolliker-Fuse nucleus. The PRG has reciprocal
connections with the medulla. Increased activity within the PRG shortens the activity of inspiratory neurons
in the medulla. Thus, inspiration will be shortened and expiration will be initiated earlier. This action is
known as phase switching and in this manner the PRG causes more breaths to occur within a given time,
thereby increasing the rate of breathing.
The lower portion of the pons exerts an excitatory influence on inspiratory neurons. Its activity is usually
suppressed by the pneumotaxic center. However, in the absence of input from the pneumotaxic center and
from lung stretch receptors breathing will stop in full inspiration. In the absence of any influence from the
pontine centers, medullary centers often generate a slow, rhythmic gasping pattern of breathing.
Groups of cells whose activity is associated with respiration are found throughout the medulla in several
different nuclei. However, the major respiratory neurons are concentrated into three recognizable groups
comprised of four major nuclei. The groups are i) Dorsal Respiratory Group (DRG) centered in the nucleus
tractus solitarius (NTS); ii) Ventral Respiratory Group (VRG) that encompasses the nucleus ambiguous
(NA) and the nucleus retroambigualis (NRA) iii) Pre-Botzinger complex (pre-BotC) which contains putative
pacemaker neurons iv) Botzinger complex (BotC) which is located in and near the nucleus retrofacialis
(NRF).
The DRG is comprised solely of inspiratory neurons. The DRG receives afferent information from
respiratory related mechanoreceptors and chemoreceptors via the ninth and tenth cranial nerves as well as
the spinal cord. Descending afferents from higher brain centers also synapse onto neurons located in the
4
Post-Baccalaureate
Jason H. Mateika Ph.D.
DRG. DRG neurons discharge immediately prior to inspiration. These inspiratory neurons are of two
types. One type (Iα) are inhibited by lung inflation and the another type are excited by lung inflation (Iβ).
DRG neurons relay their activity to phrenic motor neurons in the cervical spinal cord that control the
contraction of the diaphragm. DRG inspiratory neurons also inhibit expiratory neurons in the VRG and the
PRG.
The VRG contain inspiratory and expiratory neurons that behave in a fashion similar to the neurons that
comprise the DRG. The NA contains premotor inspiratory neurons that mainly supply external intercostal
and accessory muscles, as well as, motor neurons to the laryngeal muscles and parasympathetic neurons to
the bronchioles and heart. The rostral part of the NRA (rNRA) is comprised of inspiratory neurons while
the caudal portion is comprised of expiratory neurons (cNRA). The VRG expiratory neurons activates the
expiratory muscles (abdominal and internal intercostal) when expiration becomes active, but also sends
inhibitory activity to suppress inspiratory neurons during expiration. This ends the inspiratory phase and
contributes to termination of inspiration (inspiratory off-switch).
The BotC is rostral to the NA and is composed almost exclusively of expiratory neurons together with vagal
and glossopharyngeal motor neurons. It receives sensory input relayed via the NTS and has an inhibitory
effect on inspiratory neurons in the DRG and the VRG and spinal motor neurons (e.g. phrenic motor
neurons).
The Pre-BotC has been identified as the anatomical site of central pattern generator neurons. This region
lies caudal to the BotC and contains pacemaker neurons that generate spontaneous activity without synaptic
input. This region is discussed in more detail below (see origin of respiratory rhythm).
Control of Breathing - Patterns of Respiratory Neuron Activity
5
Post-Baccalaureate
Jason H. Mateika Ph.D.
As well as location, respiratory neurons are also classified according to their pattern of activity. As
mentioned above neurons can be classified based on whether they discharge during inspiration or expiration.
However, the pattern of discharge (firing frequency) also varies so that neurons can be classified according
to this designation. To date the firing frequency of neurons have been classified as augmenting,
decrementing or constant. Close examination of the respiratory cycle and the activity of the types of
respiratory neurons show that respiration has more than 2 distinct phase (i.e. inspiration and expiration).
More specifically, the expiratory phase of the cycle can be divided into early (E1 or post-inspiration) or late
subdivisions (also known as E2 or pre-inspiration).
Control of Breathing - Function of Respiratory Neurons
While the function of the inspiratory phase as the pump muscle driving phase is well understood, the
subdivision of the expiratory phase is not. The purpose of the post-inspiratory phase is to control airway
muscle activity so that expiratory resistance to flow prolongs inflation of the lungs to allow for better gas
mixing. The late expiratory activity simply functions to ensure that inspiration is not activated
inappropriately.
The function of a respiratory neuron is related to its axonal projections and connections. Whether the
neuron is inhibitory, excitatory, a cranial motoneuron, a pre-motor neuron, or an afferent relay neuron will
determine its connections. There are several ways of determining connections and three are illustrated
above. In (A), anatomical methods have been used to find connections. In this study, a VRG inspiratory
neuron and a phrenic motoneuron were stained with different dyes and their morphology reconstructed to
show that the medullary neuron probably projects to and synapses with the phrenic motoneuron.
Connections can also be inferred from the patterns of neuronal activity. In (B) it can be seen that phrenic
motoneuron activity coincides with that of a VRG inspiratory neuron, suggesting that the VRG neuron is the
source of the phrenic drive. While anatomical techniques can show likely connections and connections can
6
Post-Baccalaureate
Jason H. Mateika Ph.D.
be inferred from the correlation of patterns of activity, only electrophysiological techniques like crosscorrelation and spike-triggered averaging can demonstrate functional connections. In (C) the crosscorrelation of the activity of a single VRG inspiratory neuron and the phrenic nerve discharge shows a peak
after a short delay for transmission that indicates a monosynaptic excitatory connection. In (D) a triggered
average of the intracellular potential of phrenic motoneurons reveals an inhibitory post-synaptic potential,
demonstrating the inhibitory connection from the triggering Botzinger complex expiratory neuron. While
the above figure shows how connections between individual neurons may be discovered, this technique can
be extended by recording from multiple groups of neurons simultaneously using arrays of multiple
electrodes. Thus, the behavior and interconnections of large numbers of neurons can be studied.
Origin of the respiratory rhythm
and developmental changes
Much of the early work that resulted in the identification of respiratory neurons was completed in adult cats.
These findings lead to the development of a number of network models of respiratory rhythm generation
that featured mutual inhibition between populations of respiratory neurons (see above figure left- side).
However, more recently in vitro rat preparations have been used to study the activity, connection and
functions of respiratory neurons. As shown in the above figure (right side), the superfused brainstem-spinal
cord preparation has rhythmic phrenic and cranial nerve activities and a transverse medullary slice also
shows rhythmic hypoglossal nerve activity. This preparation has lead to the finding that two intrinsic
generators may be responsible for the development of the respiratory rhythm, at least in the neonatal rat.
One area in which intrinsic rhythm has been recorded is known as the rostral ventral lateral medulla
(RVLM) and the second area in which rhythmic activity has been located is known as the Pre-botzinger
complex. The intrinsic nature of the neurons in the pre-Botzinger complex is shown in the top figure on the
next page (left-hand side). That the rhythm is intrinsic is demonstrated by its persistence after blockade of
GABA and glycine inhibition with strychnine and bicuculine, respectively.
7
Post-Baccalaureate
Jason H. Mateika Ph.D.
Recent studies have examined how these two intrinsic rhythm generators interact. A typical experimental
preparation used to study this interaction is shown above (right-hand side). (A) shows that lumbar
motoneurons have a pre-I pattern of activity, and the investigators in this study traced the pathway of origin
from pre-I inspiratory neurons in the medulla to lumbar expiratory motoneurons via caudal VRG expiratory
neurons. µ-opiods were used to suppress the rhythmic drive to the phrenic nerves from the pre-Botzinger
inspiratory neurons. (B) When phrenic activity was absent, the inspiratory inhibition of abdominal
expiratory activity was also absent, demonstrating that an inhibitory connection to the RVLM pre-I neurons
must exist, probably originating from the pre-Botzinger inspiratory neurons. These experiments also
demonstrate the expiratory nature of the RVLM pre-I rhythm generator.
8
Post-Baccalaureate
Jason H. Mateika Ph.D.
The primary role of the two generators may vary with the RVLM pre-I generator being primarily
responsible for controlling hypoglossal motoneuron activity, whereas the pre-Botzinger generator assumes
control of the phrenic motoneurons. Moreover, the role of these intrinsic pacemakers may become more
defined during developmental changes. This idea is supported by recent findings which showed that
manipulation of lung inflation can produce changes in hypoglossal activity independent of phrenic activity
(see A – lower diagram on previous page). Given that this finding was in the adult, a looser coupling of the
two rhythm generators might exist in the adult compared to the neonate. This suggestion is substantiated by
recent findings, which showed that phrenic pre-motor neurons do not drive hypoglossal motoneurons,
supporting the idea of divided control functions. The idea that the two pacemakers are loosely coupled in
adults is further confirmed by findings, which show that the onset of hypoglossal nerve activity significantly
precedes the onset of phrenic nerve activity in the adult but not in the neonate where the two controllers may
be more tightly coupled (see B – lower diagram on previous page). The relative importance of the intrinsic
pacemakers may also vary between the neonate and adult, since unlike the pacemakers, interconnections
appear to have a strong influence on the breathing rhythm in adults. In other words, the respiratory rhythm
is disrupted when inhibition is blocked. The balance between the two generators in controlling the
respiratory rhythm may not only change with development but may also change with conditions. The
recording shown in C (lower diagram on previous page) was made in a 39-day old juvenile rat in situ
preparation. It shows that the change from an augmenting to a decrementing phrenic bursting pattern
appears to result from the presence of pre-inspiratory activity. In this case, the preparation had been used
for several hours and its condition was likely deteriorating. Similar effects, induced by hypoxia, have been
noted previously. So it is possible that gasping may be the result of the RVLM pre-I rhythm generator
becoming more active and dominating the control of the phrenic motor output. It has been suggested that
the RVLM rhythm generator may be the oldest, from an evolutionary point of few, and thus it may not
succumb to deteriorating conditions as rapidly as the pre-Bot. rhythm generator.
Control of Breathing - Muscles of respiration: Mechanically, pulmonary ventilation is accomplished by
altering intrapulmonary pressure, which occurs in response to contraction of the inspiratory muscles
(inspiration), and the passive recoil of the lungs (expiration). The inspiratory muscles are comprised of the
9
Post-Baccalaureate
Jason H. Mateika Ph.D.
diaphragm and the external intercostal muscles. The diaphragm is innervated by the phrenic nerve which is
formed by axons that originate from motoneurons located within the ventral horn of the C3-C5 segment of
the spinal column. The external intercostal muscle is innervated by the external intercostal nerves, which are
formed by axons that originate from motoneurons located within the ventral horn of the T1-T12 segment of
the spinal column. The expiratory muscles are comprised of the rectus abdominus and the internal
intercostal muscles. These muscles are activated at increased levels of minute ventilation. The former
responds to efferent input from motoneurons in the L1-L5 segment of the spinal column, and the latter
responds to efferent input from motoneurons in the T1-T12 segment of the spinal column. Cyclical neural
input to the respiratory muscles establishes the rhythmic pattern of breathing. This neural input originates
from the respiratory control centers that reside in the brain.
Control of Breathing - Neuronal and respiratory muscle activity during inspiration and expiration:
During normal quiet breathing (eupnea), active inspiration lasts approximately 1-2 seconds, and passive
expiration lasts 2-3 seconds, or longer. Resting respiratory frequency in adults is normally in the range of
12-15 breaths per minute. During inspiration the inspiratory neurons in the DRG and rVRG discharge and
send signals to the cervical and thoracic levels of the spinal cord. The motor neurons that receive these
signals form nerves which innervate the diaphragm and external intercostal muscles. During inspiration the
diaphragm pulls downward, the external intercostals pull the ribs upward and outward. During heavy
breathing (i.e. exercise) the accessory muscles in the neck stabilize the ribs. These muscles receive input
from cranial motor neurons located in the medulla. During quiet breathing expiration is passive. This
passive response is brought about through termination of inspiration, largely via inhibitory signals generated
by expiratory neurons located in the BotC. Signals from the BotC will inhibit inspiratory neurons in both
the medulla and the spinal cord. During passive expiration, the inspiratory muscles relax and the compliance
of the chest wall returns the ribs to their relaxed position expelling air. The expiratory neurons located in the
cNRA are primarily responsible for generating the increased drive necessary for forced expiration. These
neurons have axons which travel down to the level of the spinal cord and synapse onto motor neurons
located in the lower region of the thoracic (internal intercostal muscles) and lumbar (abdominal muscles)
spinal cord. During forced expiration the internal intercostals can pull the ribs downward and inward and the
rectus abdominis and the external oblique muscles pull the chest wall down, compressing the chest gas
volume.
10
Post-Baccalaureate
Jason H. Mateika Ph.D.
Control of Breathing - Upper airway structures: In order for air to enter or exit the lungs the upper
airway must remain open. The upper airway’s ability to remain unobstructed depends in part, on whether
the anatomy of the upper airway is compromised. The middle (MT) and inferior turbinates (IT) that are
located in the nasopharynx function to warm, clean and humidify the air as it passes through the nose and
into the lungs. If the turbinates are extremely large they can reduce or completely block airflow through the
nose. Similarly, if the tongue (T) or uvula (U) is enlarged, the upper airway could become partially or
completely obstructed. Likewise, a soft palate that is abnormally compliant (i.e. floppy) could also lead to
upper airway obstruction.
Upper airway muscles play an important role in maintaining upper airway patency. These muscles are
shown below. The function of each muscle will not be reviewed. However, note that three primary muscles
control the position of the tongue. The genioglossus, styloglossus and the hyoglossus. Contraction of the
genioglossus causes the tongue to protrude. In contrast, contraction of the hyoglossus and styloglossus
cause the tongue to retract. These muscles interact in a complex fashion to ensure that the tongue does not
obstruct the upper airway. Tonic and phasic activity can be recorded from tongue muscles. Unlike the
respiratory neurons that have axons that travel from the medulla to the spinal cord, the hypoglossal nucleus
is comprised of neurons whose axons form the hypoglossal nerve that exits at the level of the medulla and
innervates the tongue muscles.
11
Post-Baccalaureate
Jason H. Mateika Ph.D.
VENTILATION AND MECHANICS OF BREATHING
Outline:
Functional anatomy of the respiratory system
Pulmonary mechanics of the lungs and chest wall
Lung pressures and pressure gradients
Determinants of lung compliance
Lung and chest wall compliance
Dynamic lung compliance and airway resistance
Airway Diameter
Dynamic and Static Lung Volumes
Lung Disease and Static Lung Volumes
12
Post-Baccalaureate
Jason H. Mateika Ph.D.
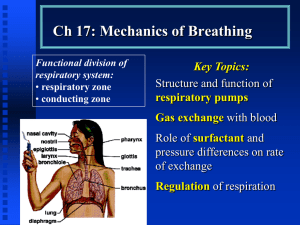
Ventilation and Mechanics of Breathing - Functional anatomy of the respiratory system: Once the
muscles of inspiration contract a negative pressure is generated. If the upper airway remains patent, air will
travel from the atmosphere into the lungs. The right lung has three lobes (upper, middle and lower) while
the left lung has two lobes. Air travels from the atmosphere to lung alveoli, where gas exchange occurs, via
the tracheo-bronchial tree. The tracheo-bronchial tree is comprised of 23 generations. The first 16
generations lack alveoli and consequently gas exchange does not occur within these generations. The first
16 generations are referred to as the conducting zone and the volume of air normally found in this zone is
referred to as the anatomical deadspace of the system. The remaining 7 zones are referred to as the
respiratory zone. Within this zone alveoli branch off from bronchioles and consequently gas exchange
occurs within the respiratory zone. Numerous branching in the respiratory zone is accompanied by a rapid
increase in the total cross-sectional area. As a result, the forward velocity of gas during inspiration is very
slow and gaseous diffusion becomes the chief mode of ventilation.
Ventilation and Mechanics of Breathing - Pulmonary mechanics of the lungs and chest wall:
PP == -5
-5
PP == 00
PP == 00
PP == 00
PP == 00
PP == 00
13
Post-Baccalaureate
Jason H. Mateika Ph.D.
The volume of air that enters the lung will depend in part on the negative pressure that is generated. This
concept is covered later in the syllabus. The volume of air that enters the lung will also depend on the
individual properties of the lung and chest wall, and the manner in which the lung and chest wall interact.
Use of a simple physical “spring” model (see lower left-hand diagram on previous page) will aid in
understanding the physical interactions between the lungs and the chest wall. The lungs are modeled as a
spring being stretched and trying to recoil back to a shorter length. This is true even at residual volume (RV)
which is the volume remaining in the lungs following maximal expiratory effort.
Alternatively, the chest wall is modeled as a spring that is being compressed and is constantly trying to
expand back to its maximum length. When the lungs and chest wall are coupled, which is normally the
case, lung volume is larger and the chest wall volume is smaller than would be the case if the lung and chest
wall were uncoupled. Consequently, the lung normally has an inward directed recoil force while the chest
wall has an outward directed force. As a result, the space between the lungs and the chest wall (the
intrapleural space) has a negative pressure (- 5 cmH2O) relative to atmospheric pressure (see lower righthand diagram on previous page). At the end of a normal expiration, the outward recoil force of the chest
wall offsets the inward recoil force of the lungs. This equilibrium state is referred to as functional residual
capacity (FRC).
There are conditions that alter the normal physical forces that govern the interaction between the lung and
chest wall. For example, the lungs can lose some of there elastic recoil in certain pulmonary diseases, such
as emphysema. When this occurs the outward force of the chest wall overcomes the inward recoil forces of
the lungs resulting in an increase in FRC. Another example is the alterations that occur following a
pneumothorax. When the chest wall becomes uncoupled from the lungs, as it often does in response to blunt
force injury, intrapleural pressure becomes the same as atmospheric pressure and the lung and chest wall
becomes uncoupled. Under this condition, the lung collapses to a minimum volume while the chest wall
expands to its maximum volume (i.e. 80 % of vital capacity). Under these conditions, mechanical
ventilation of the lungs is necessary to maintain adequate gas exchange.
Ventilation and Mechanics of Breathing – Lung pressures and pressure gradients
By convention the pressure difference is measured as inside – outside. The recoil pressure of the chest wall
(Pcw) is the difference between pleural pressure (Ppl) and atmospheric pressure (Patm). The recoil pressure of
14
Post-Baccalaureate
Jason H. Mateika Ph.D.
the lungs (PL or transpulmonary pressure) is the difference between the alveolar pressure (PA) and the
pleural pressure (Ppl). When there is no airflow (closed nose and mouth) PA and the pressure measured at
the mouth are the same. Hence, the pressure gradient across the entire respiratory system (Prs) is the
difference between the alveolar pressure (PA) and atmospheric pressure (Patm). The pressure across the
respiratory system (Prs) is also the sum of the pressure of its two components, the lung (PL) and chest wall
(Pcw).
During inspiration, the diaphragm contracts, and the chest wall and the lungs expand. As the lungs are
pulled further away from their resting position (which is below residual volume), Ppl becomes more
subatmospheric. Consequently, lung volume is increased and gas in the lungs is decompressed, and pressure
in the alveoli (PA) drops below atmospheric pressure. The created negative pressure gradient between the
airways and atmosphere generates airflow to the lungs. As inspiration proceeds, the lungs fill up with air,
and the pressure gradient and air flow gradually decrease. At the end of inspiration airflow stops because PA
is equal to atmospheric pressure (no pressure gradient). At the onset of expiration, the diaphragm relaxes,
elastic recoil of the respiratory system compresses the gas in the lungs, thereby increasing PA. The positive
pressure gradient between the lungs and the atmosphere is reversed and air from the lungs is pushed out to
the atmosphere. As lung volume decreases, Ppl slowly returns to its resting level. At the end of expiration
(i.e. at FRC) airflow and PA are 0 (ml/sec and cmH2O, respectively), and Ppl is about -5 cmH20.
Ventilation and Mechanics of Breathing – Determinants of lung compliance
The volume of air that enters or exits the lungs for a given change in pressure (∆V/∆P = compliance) will
depend in part on the properties of the lung and chest wall. We will consider the compliance of these two
structures separately prior to considering the compliance of the structures when they are coupled.
15
Post-Baccalaureate
Jason H. Mateika Ph.D.
In the experiment shown above a lung was excised and placed into an airtight container. Subsequently,
changes in lung volume and container pressure were measured. Pressure in the container was decreased and
then increased in a step-wise fashion and changes in lung volume were plotted. Decreasing pressure outside
the lung (i.e. intrapleural pressure) expanded the lung. Changes in lung volume were less at high lung
volumes for a given pressure change. When pressure was returned to atmospheric pressure lung volume
decreased, however, it followed a different path. This property is referred to as hysteresis and is the result
of changes in surface tension created from the air-fluid interface in the alveoli. The shape of the compliance
curves is determined by elastic forces caused by surface tension, and elastic forces caused by the lung.
Lung composition and compliance: Elastic behavior of the lung itself is determined by the composition and
arrangement of the collagen and elastin fibers of the lung. The construction of the lung is such that inflation
of one alveoli tends to augment the inflation of adjacent alveoli (interdependence). These tissue factors
account for about 1/3 of the compliance behavior of the lung.
Surface tension and lung compliance: The majority of static compliance behavior is determined by surface
tension. Each alveolus is an air-water interface. Surface tension is a result of unequal attraction between gas
molecules and liquid molecules. Water molecules will have more attraction for each other than for air
molecules. Thus, there is a tendency to decrease the surface area of the air water interface (to ‘contract’). In
an alveolus, this means that surface tension tends to promote deflation (collapse). Quantitatively, surface
tension is responsible for 2/3 of the compliance behavior of the lungs.
16
Post-Baccalaureate
Jason H. Mateika Ph.D.
The importance of surface tension at a gas-liquid interface is illustrated by the two static compliance curves
illustrated above. This experiment shows the effect of inflating the isolated lung with air versus saline. The
air inflated lung requires large positive pressures and exhibits hysteresis (i.e. the path followed during
expiration is different from that taken during inspiration). At low lung volumes, a greater pressure is
required to produce a volume change when the lung is inflated with air versus saline. This implies that the
air-fluid interface on the alveolar surface affects lung expansion. In contrast, the saline-inflated lung
requires much less positive pressure and exhibits no hysteresis. Note that the slope of the P-V curve (i.e.
compliance) is greater during saline inflation. This is the result of very low surface tension which will be
discussed below.
Surface tension is a force generated by the physical - chemical nature of the interaction between the air-fluid
interface in the alveoli. This force has the effect of collapsing alveoli to the smallest possible surface area.
This inward directed force accounts for approximately 2/3 of the inward elastic recoil of the lung. This
relationship is described by LaPlace’s Law (P = 2T/r) where T is the surface tension and r is the radius.
The balance between tension within the wall of the alveolus (produced by the air-fluid interface) and the
pressure inside the alveolus determines the size of the alveolus. If surface tension is the same in two alveoli
then the smaller alveolus will have a higher pressure and it will empty into the larger alveolus. If surface
tension remains constant, the pressure within the alveolus is inversely related to the radius. Thus, if a large
alveolus is connected to a small one, the gas will flow down its pressure gradient from the small alveolus to
the large alveolus. This will result in collapse of the small bubble. This condition rarely occurs in the
normal healthy lung due to the lung’s production of surfactant.
17
Post-Baccalaureate
Jason H. Mateika Ph.D.
Surfactant, surface tension and lung compliance:
Surfactant gives the alveoli a variable surface tension, so that at small volumes the surface tension is low.
Surfactant (DPPC-dipalmitoyl phosphatidyl choline) is secreted by type II epithelial cells in the alveoli of
normal lungs. The major component of surfactant is primarily a phospholipid (dipalmitoyl
phosphatidylcholine). This molecule has a hydrophobic portion and a hydrophilic portion. This serves to
orient the molecule at the air-water interface. Surfactant is a surface-active agent in water that acts to
decrease surface tension at the air-water interface of the alveolus. In doing so, surfactant i) decreases surface
tension ii) decreases work of breathing iii) increases compliance iv) stabilizes alveolar size v) dries the
alveoli
18
Post-Baccalaureate
Jason H. Mateika Ph.D.
The lower diagram on the previous page shows the effect of surfactant on alveolar stability. To understand
the diagram, we must re-consider Laplace’s Law:
P = 2T/R
P is the intra-alveolar pressure, T is the tension of the alveolus (resistance that acts to preserve the integrity
of the surface), and R is the radius of the alveolus.
In the example shown at the top of the figure (see bottom figure previous page), the alveoli are assumed to
be without surfactant. Thus, the surface tensions are equal. Based on the above equation, you can see that
the smaller alveolus will have a larger intraluminal pressure. Because gas flows from regions of high
pressure to low pressure, gas will flow from the smaller alveolus to the larger alveolus. (One alveolus will
be deflated or collapsed). When alveoli are collapsed, they are said to be atelectic.
The bottom example (see bottom figure previous page) shows the effects of surfactant on surface tension.
First, look at the alveolus on the right. Note that the presence of surfactant decreased the surface tension by
approximately 4 times. The result of this alteration is a significant decrease in the intra-alveolar pressure
from 8 cmH2O to 2 cmH2O. The importance of this change is evident when you assess the pressure in the
larger alveolus (left). In the larger alveolus, the intraluminal pressure is 4 cm H2O. Thus, gas will flow from
the larger alveolus to the smaller. The result will be two alveoli of equal size. Thus, deflation (atelectasis) is
prevented.
The bottom example shows that surface tension was lower in the small alveolus than in the large. This is
similar to what happens to surface tension in the experimental apparatus shown in the middle of the page.
Note that as the relative surface area is reduced, surface tension is decreased in the presence of surfactant.
This probably has to do with the fact that surfactant is concentrated as surface area is reduced.
Compliance of the total lung:
As stated previously compliance of the lung is defined as the change in lung volume divided by the change
in lung distending pressure (CL = ∆V/∆P).
Under normal conditions - CL = ∆V/∆P where ∆P = Palv-Ppl (for lung) and Ppl-Patm (for chest wall). For
example if ∆V = 1L and ∆P = 5 cmH2O then CL = 0.2 L/cmH2O.
19
Post-Baccalaureate
Jason H. Mateika Ph.D.
Compliance is usually measured during expiration above FRC. Compliance is increased by destruction of
the elastin and collegen content of the lung. This occurs in obstructive lung disease (i.e. emphysema).
Several factors also decrease compliance including i) high lung volume ii) respiratory diseases (restrictive
diseases) iii) alveolar edema iv) deficiency of surfactant.
Changes in pulmonary compliance in disease states:
Static pressure-volume (P-V) curves and dynamic tidal P-V loops illustrate the effects of certain respiratory
diseases on lung compliance, lung volumes and airway resistance. Airway resistance is the predominate
feature in obstructive disease (asthma, emphysema), whereas decreased compliance is most obvious in
restrictive diseases (pulmonary fibrosis and respiratory distress syndrome).
20
Post-Baccalaureate
Jason H. Mateika Ph.D.
Ventilation and Mechanics of Breathing – Lung and chest wall compliance
The interaction between the lung and chest wall can be observed if the P-V relationship of both the lung and
chest wall is plotted together. In this experiment, the subject inspires or expires into a spirometer and then
relaxes their respiratory muscles while airway pressure is measured. The points are then plotted to create
the graph above (SOLID LINE) that shows the relaxation P-V relationship. Functional residual capacity
occurs when the “relaxation pressure” of the lung and chest wall is atmospheric and the expansion force of
the chest wall opposes the recoil force of the lung. Thus, FRC is the equilibrium volume when the elastic
recoil of the lung is balanced by the normal tendency of the chest wall to expand. The DOTTED LINES
represent the P-V curves for the lung alone and the chest wall alone. If the outward force generated by the
chest wall was absent, then airway pressure at FRC would be positive owing to the elastic recoil force of the
lung. If on the other hand, the inward recoil force of the lung was absent, then airway pressure at FRC
would be negative owing to the outward expansion force of the chest wall. Note, also that the relaxation
pressure for the chest wall (i.e. atmospheric pressure) occurs at 80 % VC. This means that under “normal”
conditions the outward expansion force generated by the chest wall aids in inspiration. If the subject
increases lung volume above this point airway pressure increases. This is due to the greater inward recoil
force of the lung. The chest wall, on the other hand, is closer to where it wants to be and thus contributes
less outward elastic recoil force then at FRC. If the subject expires from FRC and relaxes then airway
pressure will be negative. This is due to the small inward recoil force of the lung and the large expansion
force of the chest wall.
Ventilation and Mechanics of Breathing - Dynamic lung compliance and airway resistance
In the previous discussion, we considered the static lung and chest wall compliance (i.e. when air is not
moving). The factors principally responsible for these measures are the elastic properties of the lung and
chest wall, and surface tension. However, we must consider compliance of the lung and chest wall when
air is moving (i.e. dynamic compliance). The additional factors that influence the dynamic compliance
measures are airway resistance, tissue resistance and inertia. Of these three, airway resistance is
quantitatively the most important.
21
Post-Baccalaureate
Jason H. Mateika Ph.D.
The above diagram shows simultaneous changes in lung volume, intrapleural pressure, gas flow, and
alveolar pressure during normal inspiration and expiration. The graph of intrapleural pressure shows the
actual pleural pressure (AB`C), and the pleural pressure required to overcome only the elastic characteristics
of the lung (ABC). The difference in pressures is due to the additional work expended to overcome
resistance to air flow.
Airway resistance can be calculated using Poiseuille’s Law. This law describes the pressure-flow
characteristics for laminar flow in a tube:
V = Pπr4
8ηl
where P is the pressure, r is the tube radius, η is fluid viscosity and l is tube length. Since resistance is
driving pressure divided by flow then we can arrange this equation to calculate resistance:
R = 8ηl
πr4
As the equation shows Poiseulle’s law predicts that resistance to laminar flow (of air or a Newtonian fluid)
is directly proportional to viscosity and tube length and inversely proportional to tube radius. Therefore,
small changes in tube radius can have large effects on resistance.
22
Post-Baccalaureate
Jason H. Mateika Ph.D.
There are a number of factors that influence airway resistance:
Branching patterns and physical dimensions of the airway (i.e. airway geometry and the impact of lung
volume on the surrounding tissue).
Airway Resistance
(cm H2O/L/sec)
Lung Volume (L)
The chief site of airway resistance is located in the intermediate-sized bronchi. Because of the increase in
cross-sectional area the small airways contribute relatively little to airway resistance. Additionally, as lung
volume increases airway resistance decreases and conductance (i.e. the reciprocal of resistance) increases.
The reduction in resistance occurs primarily because increasing lung volume provides radial traction to the
surrounding lung tissue, which decreases the possibility that the airway would collapse leading to an
increase in airway resistance.
Airway smooth muscle tone
23
Post-Baccalaureate
Jason H. Mateika Ph.D.
Airway resistance is altered by a variety of endogenous mediators. Airway smooth muscle tone is affected
by noxious stimuli that activate receptors located beneath the epithelium of smooth muscle. Axons from
these receptors travel to the medulla via the vagus nerve. Consequently, efferent fibers within the vagus
nerve will be stimulated and cause bronchial smooth muscle constriction. The parasympathetic nervous
system mediates the reflex modulated by the release of acetylcholine at muscarinic receptors on the muscle
cell. Parasympatholytic drugs (i.e. atropine or glycopyrrolate) may block the efferent response to stimulation
of this reflex arc.
The sympathetic system (shown to the far right in the above diagram) plays a role in modulating bronchial
smooth muscle tone, and some direct innervation is present as shown in the diagram. However, the
presence of circulating catecholamines is probably more important to airway resistance as direct innervation
is not extensive in most species. Agonists at the beta-2 receptor are used therapeutically to treat
bronchoconstriction (i.e. albuterol, clenbuterol).
The nonadrenergic noncholinergic system may also play a role in modulating smooth muscle tone, and
vasoactive intestinal peptide (VIP) has been shown to be a bronchodilator. Nitric oxide may also play a role
in modulation of bronchial smooth muscle tone.
A variety of inflammatory mediators can alter airway tone. Histamine and serotonin are two mediators that
can be released from mast cells in response to IgE-mediated degranulation of mast cells. Lipid mediators
{leukotrienes (LTB4), sulfidopeptide leukotrienes (LTC4, LTD4, LTE4)} and prostaglandins are also
released.
Mucus production
Airflow patterns
Airflow changes from laminar to turbulent flow when airflow velocity is increased. Even during normal
tidal breathing airflow is turbulent in the upper airways, although flow is always laminar in the small
airways (< 2 mm internal diameter). During turbulent flow, particles move in irregular and constantly
varying paths forming eddies. Resistance to airflow increases in response to turbulent flow. Flow is
turbulent when Reynold’s number is greater than 2000.
24
Post-Baccalaureate
Reynold’s number:
Jason H. Mateika Ph.D.
Re = 2rvd
η
where r is radius, v is average air flow velocity, d is density and η = viscosity .
Decreased elastic recoil of the lung
Obstructive lung disease, such as emphysema, results in loss of alveolar tissue and thus a reduction of the
inward elastic recoil of the lung. This results in collapse of small airways during expiration and increased
airway resistance.
Ventilation and Mechanics of Breathing - Airway Diameter
The loss of elastic recoil is not the only factor that may result in the collapse or compression of the airways.
Another important modifier of airway diameter is dynamic compression of airways. Dynamic compression
results in a limitation of flow during much of expiration. The graph above indicates that flow peaks early
during forced exhalation from total lung capacity and then decreases throughout the reminder of exhalation.
C shows the flow tracing during a submaximal expiratory effort. Note that over much of the trace, there is
no difference in flow compared to A (maximal effort). Likewise, when flow is forced at a relatively low
lung volume, peak flow is lower, and flow rate diminishes similar to that in A and C. Thus, gas flow during
much of expiration is limited and is independent of effort. The reason for this limitation is compression of
airways by the increase in intrathoracic pressure that occurs during exhalation.
25
Post-Baccalaureate
Jason H. Mateika Ph.D.
During inspiration, the transmural pressure gradient between the alveoli and intrapleural space (PA-Ppl) is
acting to open the airways. However, during forced expiration intrapleural pressure becomes positive and
the transmural pressure gradient is sufficiently negative at some point along the airway to cause collapse.
More specifically, the diagram in the top left corner (see bottom figure on previous page) shows pressure in
the pleural space, alveolus, intrathoracic airway, and atmosphere at FRC. Note that the transmural (or
distending) pressure is also shown for the intrathoracic airway (airway – pleural pressure). Note that the
distending pressure on the airway is +5 cm H2O at this time. During inspiration the diaphragm contracts,
pleural pressure decreases, alveolar pressure decreases, and the distending pressure on the airway becomes
more positive. These changes in pressure result in the flow of gas into the respiratory system and are
initiated by contraction of the diaphragm and external intercostal muscles. Note that the distending pressure
on the airway would tend to increase the diameter of intrathoracic airways during inspiration. At end
inspiration, the pressure tending to distend the airways is even greater, and the flow of gas has stopped
because alveolar, airway and atmospheric pressure are equal. A forced exhalation (bottom right) results in
an increase in pleural pressure and alveolar pressure. Pressure in the airway decreases as flow begins, and
the distending pressure on the intrathoracic airway becomes negative, and the airways narrow. Please note
that raising intrapleural pressure does not increase flow because driving pressure is unaltered. Maximal flow
decreases as lung volume decreases because the difference between alveolar and intrapleural pressure
decreases and airways become narrower. The relationships seen above may be exaggerated if increased
peripheral airway resistance accentuates the pressure drop in the airways. In addition, low lung volume and
disruption of the pulmonary parenchyma (i.e. emphysema) contributes to airway collapse.
Flow-related collapse occurs in larger bronchi during forced expiration. During coughing, the increased
velocity of flow results in increased turbulence (and sound). Increased velocity and turbulence will actually
aid in the clearing of secretions from the air passages. Flow-related small airway collapse occurs more
easily in certain disease states (i.e. emphysema, asthma). In these diseases, collapse of small airways may
occur and result in gas trapping.
Ventilation and Mechanics of Breathing - Dynamic and Static Lung Volumes
As discussed above, once the diaphragm contracts negative pressure is generated and the volume of air
taken into the lungs for a given change in pressure depends on the compliance of the lung and chest wall.
There are a number of lung volume measures that can be obtained both under dynamic and static conditions.
26
Post-Baccalaureate
Jason H. Mateika Ph.D.
Dynamic Lung Volumes
The above diagram shows ventilation in the lung. Inspired ventilation is comprised of deadspace ventilation
and alveolar ventilation. Upon exhalation ventilation from the deadspace and the alveolar space combine to
form total ventilation. The lung is perfused by pulmonary blood flow.
¾
Ventilation (VE) = frequency (f) * Volume (VT)
¾
Tidal vol. (VT) = dead space vol. (VD) + alveolar vol. (VA)
alveolar volume refers to the volume of air entering the alveoli only.
¾
Thus minute ventilation can also be written - VT * f = VD * f + VA * f
¾
Anatomical dead space refers to structures that do not contribute to gas exchange.
¾
Physiological dead space includes alveoli that are not perfused or poorly perfused, leading to wasted
ventilation.
27
Post-Baccalaureate
Jason H. Mateika Ph.D.
Static Lung Volumes
Simple measures of static lung volume can be obtained by having a subject completely fill their lungs and
then expire forcefully into a spirometer. The volume of air expired during such a maneuver is known as the
forced vital capacity and the volume of air expired in the first second of the maneuver is the known as
FEV1.0. These simple measures allow clinicians to diagnose preliminarily the existence of pulmonary
disease. In addition to measures of vital capacity the spirometry tracing will also allow for the measurement
of other lung volumes and capacities which are shown in the diagram below. More specifically, inspiratory
reserve volume (IRV), inspiratory capacity (IC), expiratory reserve volume (ERV) and tidal volume can be
determined via spirometry. If more sophisticated equipment is employed measures of RV can be obtained.
Once this measure is obtained total lung capacity (TLC) and functional residual capacity (FRC) can be
calculated. As noted below, measures of these volumes and capacities are important because they are
altered in response to lung disease (e.g. emphysema and bronchitis).
28
Post-Baccalaureate
Jason H. Mateika Ph.D.
Ventilation and Mechanics of Breathing - Lung Disease and Static Lung Volumes
The above table and figure show changes in lung volume that occur in response to restrictive and
obstructive lung disease. Shown in the table on the next page are examples of restrictive and obstructive
lung diseases. Note in the above table, if measures of forced vital capacity are less than normal this
reduction in volume may be indicative of lung disease. However, this reduction will not allow you to
determine whether the disease is restrictive or obstructive in nature. This is also the case if FEV1.0 is
examined solely to detect the presence of lung disease. However, obstructive and restrictive lung disease
can be differentiated by examining the FEV1.0/FVC ratio concurrently with FVC and FEV1.0. More
specifically, in obstructive lung disease FVC and FEV1.0 are reduced. In addition, the FEV1.0/FVC is reduced
because it is difficult to expire a significant portion of the FVC in the initial second because of the presence
of obstructed airways. Conversely, although the FVC and FEV1.0 are reduced in individuals with a
restrictive disease they are capable of expiring a significant percentage of this volume (80 % – 90 %) within
the initial second because the airway is not obstructed. Other lung volume alterations will also aid in
differentiating between an obstructive and restrictive disease. Note that total lung capacity, residual volume
and functional residual capacity are all increased in individuals with chronic obstructive pulmonary disease.
These lung volumes are increased in part because of the loss of lung elasticity and because air becomes
trapped in the obstructed airways. In contrast, total lung capacity, residual volume and functional residual
capacity is reduced in individuals with restrictive lung disease (e.g. pulmonary fibrosis) because the lung is
unable to inflate adequately.
29
Post-Baccalaureate
Jason H. Mateika Ph.D.
I.
II.
Example of Restrictive and Obstructive Lung Diseases
Restrictive
A.
Diseases of thoracic cage
1.
Kyphoscoliosis
2.
Ankylosing spondylitis
3.
Closed chest wall trauma
B.
Diseases of nerve supply to respiratory muscles
1.
Polimyelitis
2.
Muscular dystrophy
3.
Guillain-Barre syndrome
4.
Myasthenia gravis
C.
Abnormalities of pleura and pleural space
1.
Pneumothorax
2.
Pleural effusion
3.
Pleural thickening
D.
Pathology in lung
1.
Fibrosis
2.
Space occupying lesions, e.g. cysts
Obstructive
A.
Bronchoconstriction
1.
Asthma
2.
Inhalation of irritants, e.g. cigarette smoke
B.
Structural changes in airways, e.g. chronic bronchitis
C.
Obstructions within airways
1.
Inhaled foreign body
2.
Excess bronchial secretions
30
Post-Baccalaureate
Jason H. Mateika Ph.D.
GAS EXCHANGE
Outline:
Calculating the partial pressure of oxygen and carbon dioxide
Partial pressure of oxygen and carbon dioxide at the lung, pulmonary capillaries and
tissue
Shunts
Ventilation/perfusion mismatch
Oxygen Transport
Carbon Dioxide Transport
Acid Base Balance
31
Post-Baccalaureate
Jason H. Mateika Ph.D.
Gas Exchange: Once the diaphragm contracts negative pressure is generated and a given volume of air is
inspired into the lungs. At the level of the alveoli, gas exchange occurs in order to replenish depleted oxygen
supplies and to rid the body of excess carbon dioxide. Oxygen and carbon dioxide move between the
alveolar air and blood by passive diffusion. Gas flow (Q) is proportional to the pressure gradient (P1-P2)
divided by the resistance (R) multiplied by a constant “K”. Q = K*(P1-P2)/R (i.e. Fick’s Law). Under
normal conditions, blood is in contact with the alveoli for 0.75 seconds, and the partial pressure of oxygen
(PO2) reaches equilibrium in about 0.25 seconds. Therefore, oxygen is not normally diffusion limited.
However, if alveolar PO2 is low or the diffusion resistance is high, capillary PO2 may not reach equilibrium
with alveolar PO2. Moreover, capillary PO2 may not reach equilibrium with alveolar PO2 if pulmonary
blood flow increases to the point that blood is in contact with the alveoli for a shorter period than normal.
This latter mechanism might explain the phenomenon of hypoxemia that is observed in elite athletes during
severe exercise.
Gas Exchange – calculating the partial pressure of oxygen and carbon dioxide: Pressure is proportional
to the average force exerted by molecules colliding with the walls of a container. At sea level, the
barometric pressure is 760 mmHg conversely at high altitude (5500 meters) the barometric pressure is 380
mmHg. The barometric pressure in addition to the percent concentration of gas in the atmosphere must be
taken into account when calculating the partial pressure of a gas. The concentration of oxygen, nitrogen and
carbon dioxide in the atmosphere is 20.93 %, 79.04 % and 0.03 %, respectively. In addition, water vapor
pressure must be considered when calculating the partial pressure of a gas. When water is exposed to air,
water molecules leave the liquid and become water vapor. Vapor pressure depends on temperature, and is
independent of barometric pressure. Inspired air is warmed and saturated with water. The vapor pressure of
water at 37o Celsius (i.e. body temperature) is 47 mmHg. Thus, although the PO2 of dry air at sea level is
159 mmHg (760 mmHg* 0.2093), the PO2 once air is inspired into the lungs is 149 mmHg ((760 - 47) *
0.2093), since the water vapor pressure of 47 mmHg is subtracted from the barometric pressure. As shown
below in the table, the PO2 in the atmosphere decreases as one travels from sea level to altitude. However,
note that the decrease in PO2 occurs because the barometric pressure decreases not because of a decrease in
the % concentration of oxygen. In other words, the % concentration of oxygen remains at 20.93 %
independent of whether you are at sea level or at altitude.
ALTITUDE
0
1,000
2,000
3,000
4,000
9,000
PB(mmHg)
760
674
596
526
462
231
PO2 (mmHg)
159.2
141.2
124.9
110.2
96.9
48.4
32
Post-Baccalaureate
Jason H. Mateika Ph.D.
Gas Exchange – partial pressure of oxygen and carbon dioxide at
the lung, pulmonary capillaries and tissue bed
The above diagram illustrates the partial pressure of oxygen and carbon dioxide at the level of the lung,
pulmonary capillaries and tissue bed. The factors that alter PO2 and the partial pressure of carbon dioxide
(PCO2) in arterial blood include:
Hypoventilation
Lack of equilibration between alveolar gas and arterial blood gas
Shunts
Ventilation/perfusion mismatch
Gas Exchange – Shunts: In an ideal lung, PaO2 and PaCO2 = PAO2 and PACO2 where a = the PO2 and
PCO2 in arterial blood and A = the PO2 and PCO2 in the alveoli. In normal healthy people, these values are
close but not identical. In disease conditions, the numbers can vary greatly. The word “shunt” refers to
blood that has not undergone gas exchange that is mixed with blood that has exchanged gas. Thesbian
circulation and bronchial circulation are two natural sources of shunts. Thesbian circulation perfuses the left
ventricle and immediately dumps the blood into the left ventricle. Additionally, bronchial circulation
perfuses lung tissue and empties into the pulmonary vein. In healthy individuals, these shunts account for
approximately 2-4% of total blood flow. Perfusing collapsed alveoli or having a hole in the wall of the atria
or ventricles will produce a right to left shunt.
Gas Exchange – Ventilation/perfusion mismatch: The rate of uptake of oxygen depends on the rate at
which it is supplied (ventilation - V), and the rate at which it is removed (Q - perfusion). If all alveoli have
the same V/Q ratio, capillary PO2 will reach equilibrium with alveolar PO2, and there will be no alveolararterial PO2 difference. Conversely, V/Q heterogeneity leads to an alveolar-arterial difference. That is,
some alveoli may be hypo or hyperventilated and others may be hypo or hyperperfused. If you give a person
who has a V/Q imbalance 100% oxygen to breathe, the alveolar arterial difference may disappear.
33
Post-Baccalaureate
Jason H. Mateika Ph.D.
The alveolar oxygen and carbon dioxide levels are dependent on the V/Q ratio. The above figure provides
examples of the PO2 and PCO2 in the alveoli at extreme ends of the V/Q ratio spectrum. In one case, the
airways are completely obstructed (left-hand diagram) but the alveolus is adequately perfused with blood.
Consequently, gas exchange does not occur between the alveoli and atmosphere. Thus, the PO2 in the
alveoli decreases and the PCO2 increases compared to normal (middle diagram). In the other case, the
airways are unobstructed but the alveoli are perfused inadequately with blood. Thus, carbon dioxide does
not diffuse into the alveoli and as a result, the value of PCO2 approaches zero. Additionally, alveolar PO2
increases because gas in the alveoli equilibrates with gas in the atmosphere.
There are variations in perfusion and ventilation at different levels of the lung under normal conditions, thus
the extreme examples outlined in the previous paragraph may exist at various areas in the lung. These
variations affect the V/Q ratio and consequently PO2 and PCO2. In the upright human, the lung is
approximately 30 cm from the apex to the base, thus, gravity has an effect on blood flow distribution. The
distribution of blood flow is heterogeneous across the lung as measured using dissolved xenon. The highest
blood flow is found near the base of the lung while the lowest flow is at the apex of the lung. The uneven
distribution of blood flow in the lung is explained by the differences in the pressures in the alveoli (PA),
pulmonary artery (Pa) and pulmonary vein (Pv).
Under certain conditions, both Pa and Pv at the apex may be less then PA. When this occurs there is no blood
flow due to the lack of driving pressure gradient. This condition is referred to as zone 1; however, it
ordinarily does not occur. Further down the lung Pa is greater then PA, however, Pv is also less then PA.
34
Post-Baccalaureate
Jason H. Mateika Ph.D.
This condition is referred to as Zone 2 and the pressure gradient for blood flow is Pa – PA. In zone 3, the
driving pressure gradient is Pa – Pv since both exceed PA. Since both Pa and Pv are increasing throughout
Zone 3 the compliant pulmonary blood vessels distend passively, the vessels increase in diameter and
resistance to blood flow is decreased. In Zone 4, there is a reduction in blood flow when compared to Zone
3. This is explained by the low V/Q ratio at the very base of the lung, which leads to alveolar hypoxia and
compensatory vasoconstriction (i.e. hypoxic vasoconstriction).
Similar to blood flow, ventilation is also greater at the base of the lung compared to the apex of the lung.
The relationship between ventilation and blood flow (i.e. V/Q ratio) throughout the lung is shown in the
above diagram on the left-hand side. Note that the V/Q ratio is lower at the base of the lung compared to
the apex of the lung. This variation in the V/Q ratio will have a significant impact on the PO2 and PCO2 as
discussed previously. Thus, as shown in the above diagram on the right-hand side, the PO2 is decreased
and the PCO2 is increased closer to the base of the lung compared to measures obtained at the apex of the
lung.
Gas Exchange – Oxygen Transport: Once oxygen diffuses into the pulmonary capillaries from the alveoli,
it is transported to the tissue in order to produce energy. Oxygen is carried in two forms:
dissolved in plasma
combined with hemoglobin.
Dissolved in plasma - Henry’s law states that the amount of dissolved gas is proportional to the partial
pressure of that gas. For each mmHg of PO2, there will be 0.003 ml of O2/100 ml of blood. Therefore, at a
normal PO2 of 100 mmHg only 0.3 ml of O2 will be dissolved in the blood and transferred to the tissue. The
normal oxygen content of blood is 20 ml/100 ml of blood. Therefore, another method of transporting
oxygen to the blood must be present.
35
Post-Baccalaureate
Jason H. Mateika Ph.D.
Combined with hemoglobin - Each hemoglobin (Hb) molecule is capable of binding 4 molecules of oxygen.
When a Hb molecule binds 4 oxygen molecules than the molecule is said to be fully saturated. When fully
saturated, each gram of Hb can bind 1.34 ml of oxygen. Therefore, the oxygen content of hemoglobin
equals the hemoglobin concentration multiplied by 1.34 multiplied by the degree of saturation of
hemoglobin with oxygen.
Example: Hb content in women is 14 grams. In men it is 16 grams.
O2 content = 16 g/100 ml * 1.34 ml O2/g * 1.0 = 21.44 mlO2/100 ml of blood
The relationship between PO2 in arterial blood and the degree of hemoglobin saturated is shown in the
figure above. Focus initially on the curve labeled normal O2 affinity. Note that the relationship between
PO2 and % saturation is curvilinear. This is important because it reveals that hemoglobin will remain close
to 100 % saturated even though the PO2 decreases significantly from 100 mmHg to approximately 60
mmHg. It is only after PO2 has decreased significantly (i.e. lower than 60 mmHg) that significant
reductions in oxygen saturation occur. Next focus on the curve that is labeled decreased O2 affinity. This
curve reveals that there are a number of variables that are capable of eliciting a right-ward shift in the PO2 % saturation curve. The implications of this shift are for a given value of PO2 less hemoglobin is saturated.
Thus, any condition that results in an increase in PCO2, hydrogen ion concentration, temperature or 2,3
diphosphoglycerate will decrease the affinity of hemoglobin for oxygen. For example, carbon dioxide,
which is a by-product of metabolism, is released at the tissue level. This increase in carbon dioxide will
decrease the affinity of hemoglobin for oxygen, which allows oxygen to diffuse into the tissue to be utilized
during aerobic metabolism. Additionally, increases in temperature and hydrogen ion concentration are often
measured under conditions of extreme exercise and thus these alterations would also promote the release of
oxygen at the tissue level.
36
Post-Baccalaureate
Jason H. Mateika Ph.D.
Gas Exchange – Carbon Dioxide Transport
Carbon dioxide is carried in the blood in three forms:
Approximately 6 % of CO2 is transported in the dissolved form.
Approximately 90 % is transported in the form of bicarbonate
Approximately 4 % is transported in the form of a carbamino compound.
CO2 binds reversibly to the amino terminus of alpha and beta chains of hemoglobin. The remainder is
carried as bicarbonate (i.e. CO2 + H2O <=> H2CO3 <=> H+ + HCO3-). In a healthy individual, arterial and
alveolar PCO2 are virtually identical. Arterial PCO2 is a balance between CO2 production and elimination.
Mixed venous CO2 content is a balance between CO2 production, CO2 delivery and the CO2 equilibrium
curve.
Gas Exchange – Acid Base Balance: In addition to ensuring that carbon dioxide and oxygen levels are
maintained in arterial blood, ventilation also plays an important role in maintaining the acid-base balance in
arterial blood. More specifically, the respiratory system has a specific role in acutely altering pH in arterial
blood. Ventilation is capable of altering pH primarily via its impact on carbon dioxide. The relationship
between pH (or hydrogen ion concentration) and CO2 is revealed in the formula shown below.
[H+]
=
24 PCO2
[HCO3]
Normally
[H+] = 40 nmol/L
PCO2 = 40 mmHg
[HCO3] = 24 mmol/L
37
Post-Baccalaureate
Jason H. Mateika Ph.D.
This equation is known as the Henderson-Hasselbach equation and it simply reveals that CO2 and hydrogen
ion concentration are directly related. If CO2 increases hydrogen ion concentration will increase.
Conversely, if CO2 decreases hydrogen ion concentration will decrease.
A diagram showing the normal pH
and the changes in pH associated
with the accumulation and loss
of acids and bases
Carbonic acid
Lactic acid
Ketone bodies
Phosphoric acid
bicarbonate ions
Many conditions alter hydrogen ion concentration. As the figure above shows, the pH level is normally 7.4.
If the hydrogen ion concentration increases pH will decrease below this value leading to the development of
acidosis. Conversely, alkalosis will develop if pH increases.
38
Post-Baccalaureate
Jason H. Mateika Ph.D.
The above diagram shows specifically the role of ventilation in the control of acid-base balance. Clearly, if
there is any alteration in the respiratory system that causes an individual to ventilate above that required for
metabolism (hyperventilate) then CO2 levels in arterial blood will decrease. Consequently, the HendersonHasselbach equation reveals that hydrogen ion concentration will decrease. Although decreases in CO2 may
not be physiologically beneficial in some cases (i.e. hyperventilation is often associated with fever, anxiety,
brain disorders) it should be recognized that our ability to control CO2 via ventilation may be beneficial in
many other cases involving metabolic disorders. If arterial pH decreases because of lactic acidosis, diabetes
or diarrhea our system is capable of compensating for this change immediately by altering ventilation.
Thus, an increase in ventilation that leads to reductions in CO2 ultimately increases pH, thereby
compensating for metabolically induced increases in pH wholly or in part.
39
Post-Baccalaureate
Jason H. Mateika Ph.D.
INPUTS TO THE RESPIRATORY CONTROL SYSTEM
Outline:
Descending inputs
Ascending inputs – peripheral chemoreceptors
Ascending inputs – central chemoreceptors
Combining the efferent and afferent arms of the respiratory control loop
Other neural inputs
40
Post-Baccalaureate
Jason H. Mateika Ph.D.
Inputs to the Respiratory Control System: Inputs to the respiratory center that influence the respiratory
rhythm can be divided into descending and ascending inputs.
Inputs to the Respiratory Control System - Descending Inputs: A number of regions of the central
nervous system above the level of the brainstem are known to influence breathing. The most important of
these are the hypothalamus and the cerebral cortex.
The hypothalamus can influence breathing in at least three ways.
Changes in body temperature often lead to changes in the rate of breathing. These changes are elicited
via the hypothalamic thermoregulatory center.
Powerful emotions, such as fear, anxiety, rage etc. can profoundly affect the respiratory rhythm. These
influences arise largely in the hypothalamus.
Releasing hormones synthesized within the hypothalamus, influence breathing trophic hormones released
from the anterior pituitary. These include thyrotopin releasing hormone, which ultimately influences T3 and
T4 levels) and gonadotropin releasing hormone (which ultimately influences progesterone levels). Both
thyroid hormones and progesterone stimulate breathing.
Although the control of breathing is often involuntary it is possible to exert conscious control over
breathing. We can hold our breath or deliberately hyperventilate. In addition, the cerebral cortex has other
more subtle influences on the pattern of breathing during talking, singing and playing musical instruments.
Many descending cortical influences bypass the brainstem respiratory centers and synapse directly on
respiratory motoneurons. These voluntary pathways, originating in the pyramidal tracts, pass down the
lateral spinal columns in the corticospinal tracts. Involuntary pathways from the brainstem respiratory
41
Post-Baccalaureate
Jason H. Mateika Ph.D.
centers, on the other hand, travel in the ventral and ventrolateral columns as the bulbospinal tracts. This
separation of voluntary and involuntary control can sometimes be seen when the central nervous system is
damaged, for example following a stroke. Involuntary control may be preserved, but cannot be overruled
consciously. In other instances, involuntary control may be lost, leaving only voluntary control, as seen in
the rare condition known as congenital central hypoventilation syndrome (formerly known as “Odine’s
Curse). In addition to the hypothalamic and cortical influences, the breathing pattern is also influenced via
the “locomotory pattern generator” that relays through the thalamus. In this way, breathing can be entrained
to various locomotory rhythms (e.g. running and swimming).
Inputs to the Respiratory Control System - Ascending Inputs: The peripheral chemoreceptors are
located in the bifurcation of the common carotid arteries. They receive a very rich blood supply from a
small artery arising directly from the external carotid artery. It is claimed that the carotid bodies have a
higher blood flow per unit weight of tissue than any other structure in the body. The carotid bodies are
innervated by the carotid sinus nerve, which is a branch of the glossopharyngeal nerve (cranial nerve IX).
Each carotid body consists of two cell types. Type 1 or glomus cells are characterized by abundant
mitochondria and vesicles containing transmitter substances; these are the actual chemosensitive cells. The
type 2 cells resemble glial cells and are thought to have a supportive function. Other more diffuse groups of
cells are found in the wall of the aortic arch, and are referred to as aortic bodies. Branches of the vagus
nerve (i.e. cranial nerve X) innervate the aortic body chemoreceptors. The aortic bodies do not have a
significant role in the control of breathing in humans.
Role of the peripheral chemoreceptors: The carotid body chemoreceptors display a low level of activity at
normal levels of oxygen. This activity can be silenced by breathing increased levels of oxygen. If the blood
perfusing the carotid bodies falls, carotid body activity rises abruptly once oxygen values decline to a partial
pressure of 60-70 mmHg. It is important to understand that glomus cells in the carotid bodies respond to the
partial pressure of oxygen and not oxygen content. Thus, the carotid bodies respond to hypoxemia (e.g.
high altitude or pulmonary disease) but not to anemia or carbon monoxide poisoning. A maximal response
from the glomus cells can be elicited by infusing cyanide, which interferes with oxidative phosphorylation
within the mitochondria and hence gives the appearance to the glomus cells of total anoxia. The exact
42
Post-Baccalaureate
Jason H. Mateika Ph.D.
manner in which the receptors detect changes in the partial pressure of oxygen is not completely understood.
However, it appears to involve an increase in calcium ions in the glomus cells. It is also not completely
understood how the glomus cell signals the carotid sinus nerve. A number of neurotransmitters and
neuromodulators have been implicated. For example, dopamine levels in type I cells are altered in response
to changes in the partial pressure of oxygen. Additionally, acetylcholine (acting through both nicotinic and
muscarinic receptors) stimulates an increase in activity in the carotid sinus nerve. A number of
neuropetides, particularly adenosine and substance P also increase the activity of the carotid sinus nerve.
The carotid bodies also respond to increases in the partial pressure of carbon dioxide. Carbon dioxide is
detected by way of hydrogen ions generated within the glomus cells when carbon dioxide comes in contact
with carbonic anhydrase located in the cells. Although the carotid bodies provide only 15 – 25 % of the
hypercapnic ventilatory response, they allow for a much faster response than the central chemoreceptors.
The carotid bodies are also respond to hydrogen ions derived from other sources (i.e. lactic acid, ketone
bodies) and potassium.
Other sites of peripheral chemoreception: There has been speculation over recent years that cells in other
parts of the periphery might detect changes in local levels of oxygen, carbon dioxide or hydrogen ions. The
discovery of carbon dioxide sensitive receptors in the lungs of reptiles and birds led to studies that tried to
confirm the presence of similar cells in mammals. However, it appears that these lung receptors do not
exist, apart from the response of J receptors (see below) to hydrogen ions. Blood vessels respond to carbon
dioxide, oxygen and hydrogen ions, but this is due to the direct action of these variables on smooth muscle
in the walls of arterioles and pre-capillary sphincters. The results from studies that have been designed to
determine whether receptors that respond to oxygen, carbon dioxide or hydrogen ions exist in skeletal
muscle have not yielded unequivocal results.
43
Post-Baccalaureate
Jason H. Mateika Ph.D.
Central chemoreceptors: The central chemoreceptors are located on the ventral surface of the medulla,
where they form diffuse groupings of cells. These are somewhat remote from the medullary respiratory
center to which they are connected by afferent pathways. The immediate environment of the central
chemoreceptors is the brain extracellular fluid (ECF). Brain ECF is influenced by the metabolism of the
brain itself, the rate of flow and composition of the blood perfusing the brainstem, and the composition of
the cerebrospinal fluid (CSF). Because of the special non-fenestrated nature of the capillaries of the
cerebral circulation, the chemoreceptors are separated from the blood by the blood-brain barrier. The blood
brain barrier allows carbon dioxide to diffuse across it easily, but it excludes most ions including hydrogen
and bicarbonate ions. The properties of the cerebral spinal fluid are also important in understanding the
operations of the central chemoreceptors. The cerebral spinal fluid lacks protein, so it only has about half
the buffering power of the blood. Hence the hydration of carbon dioxide within the cerebral spinal fluid
produces excess unbuffered hydrogen ions. The pH of the cerebral spinal fluid is normally 7.3 (hydrogen
ion concentration 50 nM/L), compared with the 7.4 of arterial blood (hydrogen ion concentration 40 nM/L).
Response of the central chemoreceptors to carbon dioxide: Following a step rise in the partial pressure of
carbon dioxide there is an exponential “wash-in” of carbon dioxide across the blood brain barrier, which
begins to the affect the central chemoreceptors within about 90 seconds, reaching a steady state by about 5
minutes. The carbon dioxide influences the central chemoreceptors directly, by rapid diffusion through the
brain tissue. Due to the effective buffering of carbon dioxide within the brain extracellular fluid, this direct
effect of carbon dioxide may be transitory unless supplemented by changes in the cerebral spinal fluid,
which is in equilibrium with the blood in regards to carbon dioxide. Hydrogen ions diffuse out of the
extracellular fluid towards the chemoreceptor cells, and thus provide a second, longer lasting, stimulus.
Whichever route carbon dioxide takes to stimulate the central chemoreceptors, the receptors are responding
to the blood carbon dioxide. A decrease in the partial pressure of carbon dioxide will cause a reverse in the
movement of carbon dioxide, with the dual stimuli of carbon dioxide and hydrogen to chemoreceptor cells
being reduced. There appears to be a linear relationship between the partial pressure of carbon dioxide and
the central chemoreceptor response over the normal physiological range of partial pressure of carbon
dioxide in arterial blood.
Other central chemoreceptor locations: The location and function of the medullary chemoreceptors was
discovered approximately 40 years ago and their role in the control of breathing is well established. Very
recently there have been claims from one or two laboratories that other locations in the brain associated with
the control of respiration (solitary tract nucleus, pneumotaxic center, hypothalamus) might also contain cells
that are excited by carbon dioxide, hydrogen ions or even hypoxia. It is likely, however, that these will not
turn out to be chemoreceptors in the classic sense, but chemoresponsive relays within neural circuits
concerned with breathing.
44
Post-Baccalaureate
Jason H. Mateika Ph.D.
Inputs to the Respiratory Control System - Combining the efferent and afferent arms of the
respiratory control loop: The efferent and afferent arms of the respiratory control loop are depicted by
graphing the relationship between ventilation and carbon dioxide. Shown above is this graph. The line
labeled resting metabolic hyperbola represents the efferent arm of the respiratory control loop. The resting
metabolic hyperbola represents the impact that ventilation has on carbon dioxide. Thus, as ventilation
decreases carbon dioxide increases. Conversely, when ventilation increases carbon dioxide decreases. The
remaining lines on the graph represent the impact of the peripheral and/or the central chemoreceptors on
ventilation (i.e. the afferent arm of the respiratory control loop). For example, the line that is labeled > 150
mmHg symbolizes the impact that the central chemoreflex alone has on ventilation; since inspiring an
elevated level of oxygen abolishes the input from the peripheral chemoreceptors. Note that once a threshold
is achieved (i.e. approximately 39 mmHg) as carbon dioxide increases ventilation increases in a linear
fashion. Additionally, note that below the threshold as carbon dioxide increases ventilation does not
respond. However, even though the central chemoreceptors do not affect ventilation below a given
threshold, ventilation does not drop to zero below the threshold. Accordingly, other stimuli must be
responsible for maintaining ventilation even though the influence of the central chemoreflex is absent.
More specifically, behavioral and arousal influences (i.e. wake vs. sleep; light vs. dark; quiet vs. noise) will
maintain ventilation if the level of carbon dioxide is below the threshold. However, this statement is only
true for wakefulness. In general, under conditions of sleep, arousal and behavioral stimuli do not exist.
Thus, ventilation is not maintained if the level of carbon dioxide is below the chemoreflex threshold.
Consequently, apnea occurs, oxygen levels decrease, and carbon dioxide levels increase in the blood. The
other lines (100 mmHg, 60 mmHg and 40 mmHg) showed in the graph represent the impact of stimulating
both the central and peripheral chemoreflex. Note that the rate of raise in ventilation (i.e. slope) in response
to carbon dioxide increases as the arterial partial pressure of oxygen (PO2) decreases (100 vs. 60 vs. 40
45
Post-Baccalaureate
Jason H. Mateika Ph.D.
mmHg). Thus, the degree to which the peripheral chemoreflex is stimulated is dependent on the level of
arterial PO2. Note that the line labeled 100 mmHg represents the normal level of oxygen in arterial blood.
Moreover, note that the point at which the metabolic hyperbola intersects with the line (100 mmHg)
represents the normal resting equilibrium point for ventilation and the partial pressure of arterial carbon
dioxide (i.e. the point at which the efferent arm of the control loop is in equilibrium with the afferent arm of
the control loop). Thus, the normal resting value for ventilation is approximately 10 L/min and the resting
PCO2 is approximately 40 mmHg.
Inputs to the Respiratory Control System - Other Neural Inputs:
Proprioceptors: The brain stem continuously receives inputs from the periphery by way of ascending spinal
pathways. Many of these inputs are non-specific; others may be traced to specific peripheral receptors.
Among the most important of these are proprioceptors in muscles and joints. Increased activity in these
receptors, for example at the onset of exercise, increases respiratory activity. Although many proprioceptor
inputs come from muscle and joints in the limbs, other arise from stretch receptors and tendon organs within
the respiratory muscles themselves. The respiratory muscle proprioceptors provide important feedback to
the medullary respiratory center on the position of the chest wall.
Lung and airway receptors: As might be expected, the conducting airways and the lungs themselves possess
receptors that monitor the state of the respiratory system and alert the respiratory center to changes in a
number of variables. Many different receptors, with a wide range of properties, exist. The pulmonary
stretch receptors are found throughout the lung connective tissue. They respond to stretching brought about
by rapid and sustained inflation of the lung. Vagal afferents originating form pulmonary stretch receptors
inhibit the firing of Iα neurons and excite Iβ neurons in the DRG, thereby halting inspiration and initiating
expiration. In this way, the stretch receptors contribute to phase switching. This reflex is known as the
Hering-Breuer reflex. The Hering-Breuer reflex appears to operate on a “breath-by-breath” basis in a
number of species, but it only operates in adult humans when tidal volume becomes at least three times the
normal value (e.g. during extreme exercise or when challenged by chemical stimuli). However, in babies
the reflex is important in stabilizing the breathing pattern during at least their first year of life.
Laryngeal and tracheal receptors: These receptors are found in the mucosa lining of the larynx and trachea.
They respond to both mechanical and chemical stimuli. These receptors initiate the cough reflex, which
results in a series of forced expirations, which function to clear the airway.
Irritant Receptors: These receptors are found throughout the airway from the nose to the alveoli and as their
name imply respond mainly to irritating stimuli. Their stimulation initiates various protective reflexes,
which have stereotyped patterns depending on the location of the irritant receptors stimulated. A typical
classification is listed on the next page.
46
Post-Baccalaureate
Jason H. Mateika Ph.D.
Receptor
Reflex
Nerve
· nasal
· epipharyngeal
· laryngeal
· tracheal
sneeze
aspiration
cough
cough
trigeminal nerve
glossopharyngeal
vagus
vagus
The irritant receptors all share the ability to stimulate breathing and produce bronchoconstriction.
Bronchoconstriction is produced when bronchiolar smooth muscle is activated from the nucleus
ambiguus via the vagus nerve. The velocity of airflow during a cough (i.e. a deep breath followed by an
explosive expiration) is increased because of bronchoconstriction. Because of the increased airflow velocity
foreign particles are expelled from the airway. However, these reflexes are not always beneficial. In
asthma, bronchoconstriction is produced by the release of active agents (i.e. histamine), but these agents
also stimulate irritant receptors thereby exacerbating the bronchoconstriction via the reflex.
Pulmonary J receptors: These receptors are located within the alveolar walls, where they are able to
respond to changes either within the lung or within the pulmonary blood. J receptors increase their activity
in response to lung congestion and pulmonary edema, and are also sensitive to a range of different
chemicals, including hydrogen ions. Evidence exists to suggest that J receptors become more active
following severe exercise and on ascent to high altitudes. In either case, it is almost certainly secondary to
some degree of pulmonary edema.
47
Post-Baccalaureate
Jason H. Mateika Ph.D.
CONTROL OF VENTILATION DURING EXERCISE
Outline:
Mechanisms responsible for changes in ventilation during incremental exercise
Historical Perspective (Incremental Exercise)
Recent Perspective (Incremental Exercise)
Mechanisms responsible for changes in ventilation during constant load exercise
Phase I (Fast Response – Constant Load Exercise)
Phase II (Slow Response – Constant Load Exercise )
Phase III (Steady-State Response – Constant Load Exercise)
Post- Exercise (Recovery - – Constant Load Exercise)
48
Post-Baccalaureate
Jason H. Mateika Ph.D.
Control of ventilation during exercise - Mechanisms responsible for changes in ventilation during
incremental exercise: Shown in the diagram below are a number of inputs to the respiratory center that
might contribute to the control of ventilation during exercise. These inputs and their complex interaction
are discussed below.
Control of ventilation during exercise - Historical Perspective: Exercise, or more specially the
exercising muscle, relies on both aerobic and anaerobic metabolism for energy production (lower left
figure). When oxygen supply to active skeletal muscle is sufficient, as is the case during mild exercise,
aerobic metabolism is the primary source of energy production and the resultant carbon dioxide production
is eliminated from the blood by increased ventilation. However, when exercise intensity becomes severe
anaerobic metabolism contributes to energy production. The acid-base consequence of anaerobic
metabolism is an increase in cell lactic acid production. Bicarbonate serves to buffer lactic acid at its site of
formation in the cell. The result is an increased production of carbon dioxide by the cell. If bicarbonate
stores become severely depleted a significant increase in unbuffered lactic acid will be released from the
cell.
During mild incremental exercise oxygen consumption, carbon dioxide production and ventilation increase
in a linear fashion because energy is produced aerobically. At the onset of moderate-to-heavy exercise,
49
Post-Baccalaureate
Jason H. Mateika Ph.D.
anaerobic metabolism contributes to energy production. As outlined in the preceding paragraph carbon
dioxide production will increase in excess of oxygen consumption since lactic acid, a by-product of
anaerobic metabolism, is buffered initially by bicarbonate ions. The increase in carbon dioxide production
was initially thought to be responsible for the abrupt increase in ventilation indicated by the first dashed line
shown in the lower right diagram (see previous page). More specifically, the increase in carbon dioxide
production was thought to stimulate the peripheral chemoreceptors, which elicited an abrupt increase in
ventilation. This abrupt increase in ventilation is referred to as the anaerobic threshold or the ventilatory
threshold. Note between the two dashed lines ventilation increases linearly with the increase in carbon
dioxide production but is in excess of oxygen consumption.
At severe levels of exercise the stores of bicarbonate ions are depleted and lactic acid is not buffered.
Consequently, the amount of lactic acid buffered is reduced leading to an increase in arterial blood hydrogen
ion concentration (i.e. a decrease in pH). The increase in hydrogen ions was thought initially to enhance
ventilation further via stimulation of the peripheral chemoreceptors. The second dashed line shown in the
lower right diagram on the previous page indicates this additional increase. This additional increase in
ventilation is known as the point of respiratory compensation. Note that ventilation after the second dashed
line no longer increases linearly with carbon dioxide production. Consequently, arterial carbon dioxide
(PETCO2 in the diagram) begins to decrease below baseline values. The decrease in arterial carbon dioxide
serves to compensate for the increase in arterial hydrogen ion concentration thereby minimizing the decline
in pH.
Control of ventilation during exercise - Recent Perspective: The historical perspective focused primarily
on the roles that anaerobic metabolism and peripheral chemoreceptor activity have in the control of
ventilation during incremental exercise. However, a number of studies have revealed that abrupt increases
in ventilation may occur during exercise independent of changes in lactic acid concentration or pH. For
example, studies have shown that subjects that lack the ability to produce lactic acid (i.e. patients with
McArdles syndrome), still demonstrate an abrupt increase in ventilation during exercise that is characteristic
of the anaerobic threshold (see figure above).
50
Post-Baccalaureate
Jason H. Mateika Ph.D.
Findings in McArdles syndrome patients are similar to results obtained from healthy individuals that
complete two incremental exercise tests back to back (see figures above). During the first incremental
exercise test (upper left diagram) ventilation increases coincidently with an increase in lactic acid and a
decrease in pH. Alternatively, during the second incremental exercise test (upper right diagram) ventilation
increases abruptly even though pH is increasing and lactic acid concentration is decreasing. These findings
in McArdle’s syndrome patients and healthy controls suggests that hydrogen ion stimulation of the
peripheral chemoreceptors is likely not the only mechanism responsible for the increase in ventilation.
More, recent studies have suggested that increases in potassium, which occurs during heavy exercise, may
be responsible for increases in ventilation via stimulation of the peripheral chemoreceptors. Alternatively,
increased input from the motor cortex or exercising limbs to respiratory neurons in the medulla, or
respiratory spinal motor neurons, might be responsible for the increase in ventilation. More specifically,
during heavy exercise fast twitch muscle fibers are recruited in order to generate enough power to exercise
at a given workload. This recruitment might occur because of increased input from the motor cortex or
because of feedback from the exercising limb. This suggestion is supported by the results shown in the two
figures above which reveal that ventilation and electromyographic activity recorded from the vastus lateralis
(RMS in the figure) increased coincidently and in the presence of an increase in pH (see upper right figure).
Thus, presently most investigators would agree that a number of redundant mechanisms (each capable of
influencing ventilation if the other mechanisms are eliminated) normally interact in a complex fashion to
control ventilation during incremental exercise.
51
Post-Baccalaureate
Jason H. Mateika Ph.D.
Control of ventilation during exercise - constant load exercise:
The control of breathing during exercise represents the combined effect of several neural inputs to the
respiratory center. Neural stimuli originate from higher brain centers or from the periphery of the body.
Higher brain center mechanisms include central command, short-term potentiation and input from the
central chemoreceptors. Peripheral stimuli include stimuli from peripheral ergoreceptors, cardiac receptors,
temperature receptors, carotid sinus receptors and venous and pulmonary stretch receptors. Airway and lung
receptors and respiratory muscle receptors may also influence breathing during exercise.
Three distinct phases of ventilation can be observed under constant load light and moderate exercise; i)
phase I which is characterized by an abrupt increase in ventilation ii) phase II which is characterized by an
slow exponential increase in ventilation, and iii) phase III which is characterized by constant ventilation.
These three phases are followed by post-exercise recovery.
Control of ventilation during exercise - Phase I (Fast Response): The onset of exercise is characterized
by an abrupt increase in ventilation that occurs in response to an increase in drive from central command
and/or limb afferents. The central command drive may originate from two suprapontine structures the motor
cortex and the hypothalamus. The limb afferents that are activated immediately at the onset of exercise may
include limb proprioceptors and mechanoreceptors. This central neural input and peripheral non-respiratory
feedback stimuli continue throughout phase I, increasing ventilation so that the respiratory exchange ratio
remains unchanged despite the metabolic changes in oxygen consumption and carbon dioxide production.
However, in some cases hyperventilation occurs, pulmonary gas exchange exceeds the metabolic rate, and
as a result end-tidal measures of oxygen and carbon dioxide increase and decrease, respectively.
Control of ventilation during exercise - Phase II (Slow Response): The fast increase in ventilation is
followed by gradual increase in ventilation that occurs over a 2- 3 minute period. During this period of time
input from central command and active limb muscles continue to provide input to the respiratory control
centers. In response to inputs from the central command region and limb afferents short term potentiation of
respiratory neurons may also be activated. Short term potentiation is a central neural mechanism that
prolongs the excitatory input to the brain stem respiratory centers beyond cessation of the excitatory
stimulus. This mechanism is illustrated in the experiment shown below. In the upper panel, phrenic nerve
activity is recorded from a paralyzed ventilated cat. Subsequently, the carotid sinus nerve of the cat is
52
Post-Baccalaureate
Jason H. Mateika Ph.D.
stimulated continuously. Note that during the period of stimulation phrenic nerve activity increases
abruptly. Moreover, after the stimulus is removed phrenic nerve activity remains elevated compared to
baseline. The middle figure shows the wind-up of short-term potentiation. Rather than continuously
stimulating the carotid sinus nerve, the nerve is only stimulated during expiration (i.e. when the phrenic
nerve is silent). Note that during inspiration phrenic nerve activity is slowly increasing over time and
remains elevated after removal of the stimulus. Lastly, in the bottom figure the direct and indirect effect of
stimulating the carotid sinus nerve on phrenic nerve activity is shown. When the carotid sinus nerve is
stimulated an increase in phrenic activity occurs. During alternate periods when the carotid sinus nerve is
not activated the slow wind-up of short-term potentiation is evident.
In addition to inputs from central command, exercising limbs and short term potentiation, direct input from
the peripheral chemoreceptors may also contribute to the exponential increase in ventilation. The roles of
the peripheral chemoreceptors are more significant during heavy exercise when increases in potassium and
hydrogen ions are elevated. Both of these stimului have been shown to increase the activity of the
peripheral chemoreceptors.
Control of ventilation during exercise - Phase III (the steady-state response): During this phase
ventilation has reached a steady-state. Historically, it was postulated that the mechanisms responsible for
phase I and II of exercise interact in an additive fashion to produce phase III. This theory, first proposed by
Dejours in 1964, is known as the neuro-humoral theory. However, based on recent findings it is likely that
the mechanisms outlined above interact in a more complex fashion.
Control of ventilation during exercise - Post- Exercise (Recovery): There are often two phases to the
recovery period. At the end of exercise, ventilation often decreases abruptly. This abrupt decrease may
mirror the cessation of central command drive and muscle afferent feedback. Following the abrupt
decrease, ventilation usually declines in an exponential manner. This slow decline may mirror the gradual
cessation of short-term potentiation, and the removal of metabolic products such as hydrogen and potassium
ions.
53