1-1 Alveolates - Instituto de Higiene
advertisement
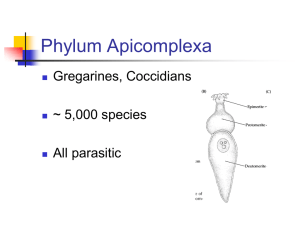
Alveolates Secondary article Article Contents Michael A Sleigh, University of Southampton, Southampton, UK . Outline Description and Characterization Three groups of Protozoa, the ciliates, dinoflagellates and sporozoans have been grouped together as ‘alveolates’ because typical cells in all three groups have a pair of subsurface membranes, forming inflated or flattened alveoli (fluid-filled cushions), beneath the surface membrane. The close relationship between the groups has been confirmed by molecular sequence analysis. Outline Description and Characterization The name alveolates has been given to a cluster of three large groups of protozoa, the ciliates (Ciliophora), Dinozoa (dinoflagellates) plus a few species with atypical features) and Sporozoa (more or less equivalent to Apicomplexa), which in many ways are very different from one another. In former schemes of classification these three groups were placed far apart, but the distinctive feature which they share appears to be a true ancestral character, since phylogenetic analysis by ribosomal ribonucleic acid (rRNA) sequencing has shown that the three groups are more closely related to one another than any one of them is to any other protist group (Gajadhar et al., 1991). All three groups have an unusual arrangement of membranes at the cell surface in most members and at some stage in their life cycle. These membranes form a system of alveoli over much of the surface in many ciliates and dinoflagellates, forming part of the pellicles which characterize these two groups, but are pressed together as a sandwich of three membranes in the Sporozoa. Ciliates like Paramecium and Tetrahymena were among the first cells whose fine structure was studied with the electron microscope. Sections of these cells showed that over much of the body the surface membrane was underlain by two inner membranes separated by a fluidfilled space. It was soon discovered that the inner membranes actually belonged to cushion-shaped alveoli, regularly arranged between the rows of cilia of the cell surface, so that cilia and trichocysts could emerge in bands between the alveoli where only a single membrane was present (Figure 1a). These alveoli occasionally contain plates of glycoprotein which may be calcified. Comparable examination of the cells of dinoflagellates like Ceratium and Peridinium showed that the thecal plates which cover these cells are actually situated internally, within alveoli which lie under the surface membrane (Figure 1b). The fine structure of sporozoan cells varies with the stage of the life cycle, and tends to be simplified in intracellular stages of these parasites. In the extracellular stages of most species the surface membrane of much of the cell is underlain by two further membranes, tightly pressed together without . Phylogeny and Place in Overall Taxonomic Scheme . Major Subtaxa and Well-known Species any space between (Figure 1c). In all three groups the triple membrane structure tends to be underlain by a layer of granular cytoplasm containing microtubules, so that comparison of pellicular structure led to the suggestion that the membrane sandwich of the sporozoans probably arose from flattened internal alveoli beneath the surface membrane. All three groups that comprise the alveolates have had an extensive evolutionary radiation, in each case dependent upon the development of a distinctive pattern of organization which is unique in the living world. It is estimated that there are about 7500 species of ciliates, 2000 living species of dinoflagellates (as well as a similar number C C E (a) (b) M (c) Figure 1 Diagrams of sections through the surface of members of the three alveolate groups to show the arrangement of membranes. In ciliates (a), rows of cilia (C) and extrusive organelles (trichocysts or mucocysts) (E) emerge between the alveoli which underlie the surface membrane. In dinoflagellates (b), thecal plates may occupy the pellicular alveoli. In sporozoa (c), the two inner membranes are pressed tightly together without any fluid space between, though these inner layers are interrupted at pits called ‘micropores’ (M). ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 1 Alveolates of fossil species, due to the traces left by the hard theca in some forms) and 5000 species of sporozoans. Characteristics of the three main groups Ciliates The ciliates are diploid cells, typically with two types of nuclei, one or more polyploid macronuclei as well as one or more diploid micronuclei, and with only the latter participating in the exchange of genetic material during the sexual process of conjugation. They also have a cortical structure in the pellicle, including the highly specific arrays of cilia and their associated specific patterns of rootlet fibres (the infraciliature) as well as the system of alveoli, which is passed on to daughter cells through a mechanism of nongenetic, somatic inheritance. The majority of ciliates are free-living cells, actively swimming with their cilia and feeding on other microorganisms filtered from the water or picked up from surfaces. Food particles are ingested through a special area of the body surface, the cytostome, and digested in food vacuoles. Contractile vacuoles for salt and water balance are best developed in ciliates. The parasitic forms typically live in the gut of their host, and usually retain distinctive ciliate features. Dinoflagellates Most dinoflagellates are haploid cells whose nucleus contains obvious condensed chromosomes throughout the cell cycle. This is because the chromatin of dinoflagellates contains little histone protein, a feature that led to the suggestion that dinoflagellates were ‘mesokaryotic’ organisms that belonged part way between the prokaryotic bacteria with no histones and the eukaryotes with many histones. However, dinoflagellates are true eukaryotes in other respects, with mitotic and meiotic nuclear divisions and fertilization. They are typically biflagellate cells, generally with one longitudinal, backwardly-directed flagellum and one transverse flagellum which executes a helical beat, often within an equatorial groove. The pellicle generally has a pattern of alveoli (called amphiesmal vesicles by workers on this group) which appear empty or contain thin plates in the ‘naked’ or ‘unarmoured’ species but contain thick thecal plates in the thecate or ‘armoured’ species. The plates are principally composed of polysaccharide material and impose specific shapes upon thecate forms. About half of the species contain brown plastids and are photosynthetic. Most colourless species, as well as some which contain plastids, engulf other microorganisms at a naked area near the flagellar bases; some species produce, from the naked area, pseudopodia which extend to form a thin protoplasmic veil around diatoms larger than themselves and digest them within a food vacuole outside the main body. There is a considerable diversity of body form, in free-living forms as well as in parasites in higher animals and photosynthetic symbionts 2 in corals and clams, and some species have unusual internal organelles. Where sexual reproduction occurs, fertilization involves the fusion of two small biflagellate gametes. Sporozoans The sporozoans are haploid parasitic protozoa, mostly with complex life cycles usually involving at least one stage when the parasite grows within a host cell. Although the traditional name of Sporozoa has remained widely used and understood, the principal subgroup was given the new name Apicomplexa a few years ago, in recognition of the fact that there is a distinctive group of organelles at the apical end of specific stages (those capable of penetrating host cells) in the life cycle of most species. This apical complex of polar organelles consists of the conoid, a truncated cone of short spiral microtubules, through which pass the stalks of secretory organelles called rhoptries, and around which there are two rings, one circling the distal end and the other circling the proximal end of the conoid. The apical complex is a site of attachment of the sporozoan to a host cell, and the rhoptries are believed to release products that stimulate the host cell to invaginate and draw in the parasite. In many cases hosts are infected with sporozoans by ingesting spores; these spores develop in the gut of the host to release spindle-shaped infective cells called sporozoites, which enter cells of the host. Similar sporozoites are injected into certain hosts by vector organisms, like the mosquito when it transmits the malarial parasite. Parasite growth and (usually repeated cycles of) reproduction take place before a process of fertilization between two parasite cells occurs, their nuclei fuse, and subsequent meiosis is followed by spore and/or sporozoite formation. In some cases the gamete cells which fuse are similar amoeboid cells, and in other cases a flagellate microgamete swims to fuse with a stationary macrogamete. Phylogeny and Place in Overall Taxonomic Scheme Until recently the three groups that comprise the alveolates were classified separately, and rather far apart. The ciliates were generally regarded as the most advanced of the protozoa, largely because many of them are among the most complex cells known. Although Haeckel in 1866 included ciliates (as Infusoria) in the Animal Kingdom, they were regarded as a separate class in the phylum Protozoa in Bütschli’s 1880 classification and as a separate phylum in the kingdom Protozoa by Levine et al. in 1980. The Sporozoa were likewise a separate class of the Protozoa in 1880 and a separate phylum (Levine’s Apicomplexa) in 1980. With their haploid and rather simpler cells, the Sporozoa were regarded as more lowly protozoans in most systems. Dinoflagellates were claimed as algae by phycologists, and this was widely accepted ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net Alveolates because the most familiar species were brown photosynthetic cells, an observation which probably led Bütschli to place them near diatoms. On the other hand many dinoflagellates feed as animals, so that protozoologists included them in protozoan classifications as members of the subphylum Mastigophora. However, in the 1970s and 1980s structural similarities between features of ciliates and dinoflagellates were already leading to suggestions that these groups were probably more closely related than had previously been believed. The possibility that the membrane arrangement of Sporozoa might reflect a common ancestry with ciliates and dinoflagellates made it easier to accept the evidence from the similarity of sequences of small subunit rRNA, which placed the three groups together on a separate branch of the phylogenetic tree. The recent confirmation that sporozoan cells contain relict organelles containing plastid-like, circular deoxyribonucleic acid (DNA) (Wilson, 1998), gives rise to the possibility that the ancestor of all alveolates may have been photosynthetic. In the universal phylogenetic scheme there appear to have been a number of early branches from the main trunk of eukaryote evolution, including Archezoa and Euglenozoa, before a wide radiation of protists towards the crown of the tree gave rise to alveolates and to some other groups of flagellate and amoeboid Protozoa as well as the kingdoms Chromista, Fungi, Animalia and Plantae. In a recent revision of his classification of the kingdom Protozoa within the empire Eukaryota, Cavalier-Smith (1998) placed the three main groups of alveolates in the following manner: Kingdom Protozoa Subkingdom Eozoa Infrakingdom Alveolata Superphylum Miozoa (a) (b) Phylum Dinozoa Phylum Sporozoa Superphylum Heterokaryota Phylum Ciliophora Major Subtaxa and Well-known Species The phylum Dinozoa A series of comprehensive reviews on features of dinoflagellates can be found in the book edited by Taylor (1987). At that time dinoflagellate taxonomy was stated to be ‘in a greater than usual state of flux’, and a number of genera remained unclassified. Some of these have been placed in the subphylum Protalveolata by Cavalier-Smith (1998), including the common marine Oxyrrhis (Figure 2a) and some other forms, all of which have typical eukaryote chromosomes. The remaining dinozoans are placed in some 14 orders in the subphylum Dinoflagellata. Knowledge of the group is limited by the fact that, with a few notable exceptions, research on the nonphotosynthetic members, and particularly the parasitic forms, has not been well integrated with that on photosynthetic forms. This probably depends on the general view among zoologists that dinoflagellates were the province of botanists, and a general lack of interest among botanists in parasites that live in animals. Photosynthetic dinoflagellates are found abundantly in the plankton. Almost all freshwater dinoflagellates are motile autotrophic forms belonging to such thecate genera as Ceratium and Peridinium (Figure 2b), and naked forms like Gymnodinium and Amphidinium, with rather typical dinoflagellate shapes, which live in lakes, and can form (c) (d) Figure 2 Examples of the Dinozoa. (a) The protalveolate Oxyrrhis (about 25 mm long). (b) Peridinium with a typical dinoflagellate shape and thick thecal plates (cell about 50 mm long). (c) In Ornithocercus the thecal plates are produced into wide flanges around the flagellar groove and at the posterior (cell about 100 mm long). (d) Prorocentrum (about 50 mm long) lacks an equatorial flagellar groove, but still has flagella which beat in different patterns. ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 3 Alveolates blooms under favourable conditions of nutrients, light and temperature. Marine dinoflagellates are more diverse; marine species of the same four genera are joined by similar forms like Gonyaulax and by forms like Dinophysis and Ornithocercus (Figure 2c), with deep thecal flanges between which the transverse flagellum moves, and Prorocentrum (Figure 2d), with no transverse groove at all. Species of Protoperidinium, Ceratium and Gyrodinium, among others that contain chloroplasts, are also known to ingest particulate food, and about half of all dinoflagellate species are heterotrophs that have no chloroplasts; these include species in genera like Protoperidinium and Gyrodinium, many of whose members are photosynthetic. Photosynthetic dinoflagellates have importance beyond the fact that they are among the principal primary producers in the plankton. Many species form conspicuous blooms, containing millions of cells per litre, referred to as ‘red tides’ (in a minority of cases other types of flagellate, or even the ciliate Mesodinium, may be responsible for the red water). Such blooms create problems for other aquatic organisms, killing fish and other animals; these problems may be of a more general nature when they result from the depletion of oxygen by bacteria in the water where dead dinoflagellate cells are decaying. A few of the bloomforming dinoflagellates (e.g. Gymnodinium breve) are directly toxic to fish at levels below those which severely deplete oxygen levels, but more important are the dinoflagellate toxins which are accumulated by filterfeeding bivalve molluscs, and indirectly kill animals that eat the shellfish. Toxins (saxitoxin and gonyautoxins) from several species of Gonyaulax, especially G. tamarensis, and Pyrodinium can accumulate to sufficient levels in the tissues of clams, mussels and oysters to cause severe cases of paralytic shellfish poisoning in humans by blocking sodium fluxes through neuromuscular membranes. Another type of toxin accumulated in shellfish, and thought to come from species of Prorocentrum, has caused diarrhoetic shellfish poisoning in Japan and Europe. It is thought that ciguatera toxins in the moray eel and some other fish may also come from dinoflagellates. A few species of photosynthestic dinoflagellates live in vaculoes within the cells of marine protozoa and animals, including radiolarians, acantharians, foraminiferans, cnidarians (jellyfish, sea anemones and corals), flatworms and molluscs (clams) (Smith and Douglas, 1987). At one time all of these symbiotic dinoflagellates were referred to as ‘zooxanthellae’; it is now believed that most of them belong to one species, Symbiodinium microadriaticum, but a few hosts contain other symbionts (e.g. species of Amphidinium). Both partners in the relationship benefit because varied products of photosynthesis are released by the symbiont and used by the host, and nitrogen and phosphorus compounds released by the host are used by the symbionts. In addition it is believed that the photosynthetic activity of the symbiont accelerates calcium 4 carbonate deposition in the skeletons or shells of organisms such as corals and foraminiferans. Parasitic dinoflagellates often bear little resemblance to their free-living relatives, but betray their relationship by the structure of the motile dinospores, which have a characteristic dinoflagellate appearance. Three principal groups were recognized by Cachon and Cachon (1987): one extracellular and living on other protozoa, invertebrates and fish (e.g. Blastodinium, Oodinium); a second living entirely intracellularly, usually in radiolarians, but sometimes in crustaceans and in fish eggs (e.g. Syndinium, Ichthyodinium); and in a third the parasite starts to grow within the cells of its host, usually a protozoan, and then emerges as a worm-like body before dividing into dinospores (e.g. Amoebophrya). Some of the fish parasites in the first group cause economic damage in aquaculture, and damage to crustacean fisheries has also been attributed to dinoflagellates. The phylum Sporozoa The composition of this entirely parasitic phylum has recently been revised and broadened by Cavalier-Smith (1998) under the name Sporozoa, which includes groups formerly classified in the Apicomplexa. For some time an oyster parasite Perkinsus was regarded as a (possibly primitive) apicomplexan, because it has parts of the apical complex and pellicular alveoli, but it also has features of dinoflagellates, and has been transferred by CavalierSmith to the Protalveolata group of the Dinozoa. Three classes (or subphyla) of sporozoans, the Gregarinidea, the Coccidea and the Haematozoea, have been recognized for a long time, although some authors retain the Haematozoea and Coccidea within a single class, as is the case in the widenened scope of the coccidian group in Cavalier-Smith’s recent revision. Like other parasites, sporozoans must reproduce very effectively in order to ensure enough progeny to infect a new host; they may show reproduction in some or all of the following three stages: by mitotic division to increase the infection within a host (schizogony); by mitotic division in the formation of numerous gametes (gametogony); and following fertilization by divisions that include meiosis in the formation of infective sporozoites (sporogony). All sporozoans commence their parasitic life with an intracellular stage resulting from the entry of a sporozoite into a host cell (Kreier and Baker, 1987). After this initial intracellular growth, gregarines outgrow the space available within the host cell and escape to live and grow further within the digestive tract or body cavity of their invertebrate or lower chordate host, before joining in pairs within a common membrane and producing large numbers of gametes. Fusion of paired gametes is followed by sporogony forming sporozoites within sporocysts, resting stages which infect a new host by being eaten. Generally the ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net Alveolates (a) (b) Figure 3 Examples of Sporozoa. (a) Extracellular gregarine cells like this Gregarina from the gut of a mealworm may reach lengths of 200 mm or more. (b) Coccidians of different genera form characteristic spores, as shown by this Eimeria in which the outer oocyst (35 mm long) contains four sporocysts, each containing two sporozoite cells. most extensive multiplication of gregarines takes place during gametogony. Well-known examples are Monocystis in earthworms, Selenidium in marine annelids and Gregarina (Figure 3a) in insects. Most coccidians gain entry to their hosts by being eaten as sporocysts, from which sporozoites emerge to infect, typically, cells of the gut wall. After entry to cells these sporozoans remain intracellular, generally going through many cycles of asexual reproduction (schizogony), bursting the host cells to release many infective merozoites which enter new cells within the same host. Eventually the parasite cells mature into gametes, one macrogamete being formed per host cell or many flagellate microgametes. The microgametes escape and swim to fertilize macrogametes; after fertilization the zygote divides a few times, with meiosis, to form sporozoites within sporocysts (Figure 3b). Multiplication here takes place principally by schizogony. This group contains important parasites in the genus Eimeria, long known to be responsible for coccidiosis in domestic animals and birds. There are several coccidians that cause serious disease in immunocompromised patients, although others seem to carry them without illness. Among these is Toxoplasma, which has a high human prevalence but does not undergo a complete life cycle in humans, although it develops as a typical coccidian, similar or identical to Isospora, in cats. Sarcocystis, named from its cysts found in sheep, and occasionally, human, muscle, is a related Isospora, with a sporogony stage in dogs. Cryptosporidium, whose spores find their way into drinking water, and which has caused recent health scares, is another coccidian. The group also includes some blood parasites which have coccidian features in their life cycle; these include Haemogregarina and Lankesterella, found in lower vertebrates, but with vector stages in invertebrates that are usually eaten by the vertebrate, although some inject parasites with saliva. The apical complex of infective cells of haematozoeans lacks the conoid or polar rings. The sporozoites develop in blood-sucking arthropods and are injected as naked cells into the vertebrate host in saliva. The sporozoites enter cells of the vertebrate host, grow and undergo repeated stages of schizogony, mostly, but not exclusively, in blood cells. Eventually parasite cells prepare to form gametes, but will not mature into gametes unless they are taken into the gut of a blood-sucking vector. Within the vector gut the parasites form macro-or microgametes, performing fertilization to form a migratory zygote, whose growth and sporogony produces many sporozoites. Members of both orders of haematozoeans are important parasites. Those in the order Haemosporida are transmitted by dipteran flies. The best known examples of this group are malarial parasites belonging to the genus Plasmodium, several species of which are transmitted between humans by mosquitoes; other species are found in other mammals and birds. Leucocytozoon and Haemoproteus are genera found in birds and reptiles and transmitted by midges and blackfly. Important members of the order Piroplasmida, which are transmitted by ticks, belong to the genera Babesia and Theileria. These are responsible for serious diseases of domestic animals throughout the world, and generally only cause human disease in cases of immune deficiency or removal of the spleen. Cavalier-Smith (1998) has included within the Sporozoa several genera of other spore-producing protozoa (Paramyxa, Haplosporidium and Metchnikovella), formerly classed elsewhere in independent groups, or as microsporidians. These are parasites of invertebrates; their phylogenetic relationships remain to be firmly established. The phylum Ciliophora The characteristics of ciliates and the composition of this phylum have been comprehensively described by Corliss (1979). They have been classified in many different ways, generally on the basis of the arrangement of cilia on the general body surface and in the region of the cell mouth (cytostome). In a recent scheme Corliss (1994) divided this phylum into eight classes, following ultrastructural studies and extensive discussion by many authors. This scheme is likely to change when the results of rRNA analysis are also taken into account; at present the molecular sequences of too few species have been studied (Hirt et al., 1998) to propose more than small changes to the scheme put forward by Corliss. Members of the class Karyorelictea are probably the most primitive surviving ciliates and are unusual in possessing nondividing, diploid macronuclei and generally lacking pellicular alveoli; they are often long, but flattened, ciliates, mostly from marine sands. Within the class Polyhymenophora, all of which have a conspicuous row of large membranelle cilia associated with the cytostome, ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 5 Alveolates P M M (b) (c) C (d) (a) (e) Figure 4 Examples of the Ciliophora. (a) Stylonychia (about 200 mm long) with a row of membranelles (M) and other cilia formed into bundles as cirri (C). (b) Strombidium (about 30 mm long) with a short band of feeding membranelles (M). (c) Didinium (about 100 mm long) with a ‘proboscis’ (P) for feeding and two bands of swimming cilia. (d) The suctorian Podophrya (about 50 mm in diameter) with tentacles for feeding and an attachment stalk. (e) The soil ciliate Colpoda (about 75 mm long). the subclass Heterotrichia, which also have a full body covering of ciliary rows, appear from rRNA analysis to be closely related to karyorelicteans. Members of several genera of heterotrich ciliates are commonly encountered, probably the best known are the large species of the trumpet-shaped Stentor and the cylindrical Spirostomum, which commonly reach 2 mm in length; Metopus species are anaerobes and Nyctotherus is a common symbiont in the gut of amphibians. The other subclass of polyhymenophorans are the Spirotrichia, apparently more-evolved ciliates that have few body cilia, often grouped into compound cirri; this group includes several well-known ciliates that ‘run’ around over surfaces, e.g. Stylonychia (Figure 4a), and Euplotes and Aspidisca, both of which are common in fresh and salt water and important consumers of bacteria in sewage treatment works, as well as important planktonic ‘oligotrich’ ciliates like Strombidium (Figure 4b) and the tintinnids, whose cup-shaped or cylindrical loricas are abundant in plankton samples. A second group at this intermediate level of evolution, according to rRNA evidence, is the class Litostomatea, including forms with very simple, or even lacking, mouth cilia; it includes Balantidium, the only ciliate parasitic in humans, ciliates which inhabit the rumen of ruminant mammals and the hind-gut of other herbivores like horses, as well as active predatory ciliates like Didinium (Figure 4c) which preys on species of Paramecium. The first group to separate at the more advanced level of evolution appears to be the class Phyllopharyngea, characterized by the possession of ribbons of microtubules around the cytopharynx leading into the cytoplasm from the cell mouth, e.g. Chilodonella or chonotrichs, or from the multiple mouths of the suctorians, e.g. Acineta, 6 Podophrya (Figure 4d). Some forms have a prominent cytopharyngeal basket of microtubular rods, which is also present in a related class, the Nassophorea, e.g. Nassula, some of which ingest filamentous algae. Close to these two classes is the class Colpodea, most of whom, like the ubiquitous Colpoda species (Figure 4e), are found in soils, at least as cysts. Most of the remaining ciliates belong to the class Oligohymenophorea, named from the presence of a small number (3–4, commonly) of membranelles or comparable compound cilia around the mouth, and rows of body cilia. Among the six subclasses, the Hymenostomatia, e.g. Tetrahymena, the protozoan most studied by biochemists, the Peniculinia, e.g. the familiar Paramecium species and the Peritrichia, e.g. Vorticella and Carchesium, well-known and extremely important bacterivores in sewage treatment systems, all have important places. Of the other three subclasses, the Astomatia are endoparasites, mostly in annelids and the Apostomatia are mostly ectoparasites on crustaceans, but the Scuticociliatia are often abundant bacterivorous forms with few cilia, e.g. Uronema, Pleuronema. The final class, the Prostomatea, contains ciliates with an anterior mouth and simple mouth ciliature, which are often predatory or detritivores. References Cachon J and Cachon M (1987) Parasitic dinoflagellates. In: Taylor FJR (ed.) The Biology of Dinoflagellates, pp. 571–610. Oxford: Blackwell. Cavalier-Smith T (1998) Neomonada and the origin of animals and fungi. In: Coombs GH, Vickerman K, Sleigh MA and Warren A (eds) Evolutionary Relationships Among Protozoa, pp. 375–407. London: Chapman and Hall. ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net Alveolates Corliss JO (1979) The Ciliated Protozoa: Characterization, Classification and Guide to the Literature, 2nd edn. Oxford: Pergamon. Corliss JO (1994) An interim utilitarian (‘user friendly’) heirarchical classification of the protists. Acta Protozoologica 33: 1–51. Gajadhar AA, Marquardt WC, Hall R et al. (1991) Ribosomal RNA sequences of Sarcocystis muris, Theileria annulata and Crypthecodinium cohnii reveal evolutionary relationship among apicomplexans, dinoflagellates and ciliates. Molecular and Biochemical Parasitology 45: 147–154. Hirt RP, Wilkinson M and Embley TM (1998) Molecular and cellular evolution of ciliates: phylogenetic perspective. In: Coombs GH, Vickerman K, Sleigh MA and Warren A (eds) Evolutionary Relationships Among Protozoa, pp. 327–340. London: Chapman and Hall. Kreier JP and Baker JR (1987) Parasitic Protozoa. Winchester, MA: Allen and Unwin. Sleigh MA (1989) Protozoa and Other Protists. Cambridge: Cambridge University Press. Smith DC and Douglas AE (1987) The Biology of Symbiosis. London: Edward Arnold. Taylor FJR (ed.) (1987) The Biology of Dinoflagellates. Oxford: Blackwell. Wilson RJM (1998) Plastid-like DNA in apicomplexans. In: Coombs GH, Vickerman K, Sleigh MA and Warren A (eds) Evolutionary Relationships Among Protozoa, pp. 293–304. London: Chapman and Hall. Further Reading Anderson OR (1987) Comparative Protozoology. Ecology, Physiology, Life History. Berlin: Springer. Coombs GH, Vickerman K, Sleigh MA and Warren A (eds) (1998) Evolutionary Relationships Among Protozoa. London: Chapman and Hall. Grell KG (1973) Protozoology, 3rd edn. Berlin: Springer. Hausmann K and Hülsmann N (1996) Protozoology, 2nd edn. Stuttgart, Germany: Thieme. Kudo RR (1996) Protozoology, 5th edn. Springfield, IL: Thomas. Lee JJ, Hutner SH and Bovee EC (eds) (1985) An Illustrated Guide to the Protozoa. Lawrence, KS: Society of Protozoologists. Levine ND (1988) The Protozoan Phylum Apicomplexa. Boca Raton, FL: CRC Press. Puytorac P de, Grain J and Mignot J-P (1987) Precis de Protistologie. Paris: Boubée. Sleigh MA (1989) Protozoa and Other Protists. Cambridge: Cambridge University Press. ENCYCLOPEDIA OF LIFE SCIENCES / & 2002 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 7