Chem 2 AP HW 9
advertisement

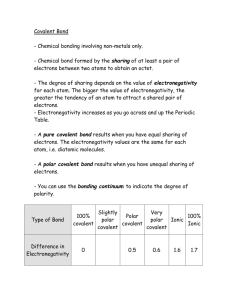
Homework 9-2: Electronegativity and Lewis Dot Structures p.379 #20, 30, 33, 34, 36, 40, 44, 45, 47, 48 20 For each of the following pairs of elements, state whether the binary compound they form is likely to be ionic or covalent: (a) B & F: Covalent (BF3, boron trifluoride) (b) K & Br: ionic (KBr, potassium bromide) 30 How many lone pairs are on the underlined atoms in these compounds: HBr: Br has 3 lone pairs H2S: S has 2 lone pairs CH4: C has no lone pairs 33 Define electronegativity, and explain the difference between electronegativity and electron affinity. Describe in general how the electronegativities of the elements change according to position in the periodic table. Electronegativity: the ability of an atom to attract electrons to itself when bonded to another atom. Electron Affinity: the amount of energy released when an electron is added to an atom. ** Both give a measure of how much an atom attracts electrons, but EA is a thermodynamic quantity based on an atom in the gas phase, and EN describes the interaction between two atoms. Electronegativity increase as one moves across a period from left to right (smaller atoms, so attract electrons more.) and decreases as one moves down a column (larger atoms, so attract electrons less.) 34 What is a polar covalent bond? Name two compounds that contain one or more polar covalent bonds. Polar Covalent Bond: a covalent bond where electrons are not shared equally. Ex: HCl, H2O, NH3 36 Arrange the following bonds in order of increasing ionic character Determine ΔEN = electronegativity difference. The bonds arranged in order of increasing ionic character are: C−H (ΔEN = 0.4) 40 < Br−H (ΔEN = 0.7) < F−H < Li−Cl (ΔEN = 1.9) (ΔEN = 2.0) < Na−Cl (ΔEN = 2.1) < K−F (ΔEN = 3.2) Classify the following bonds as ionic, polar covalent, or covalent, and give your reasons: (a) Si-Si: The two silicon atoms are the same. The bond is covalent. (b) Si-Cl: The electronegativity difference between Cl and Si is 3.0 − 1.8 = 1.2. The bond is polar covalent. (c) CaF: The electronegativity difference between F and Ca is 4.0 − 1.0 = 3.0. The bond is ionic. (d) N-H: The electronegativity difference between N and H is 3.0 − 2.1 = 0.9. The bond is polar covalent. 44 Write Lewis structures for the following molecules and ions: H (a) (d) F − O O F H (b) F (e) N H N F H O C Cl C (c) H O − H Si Si H H (f) H H H H C N H H + H 45 Write Lewis structures for the following molecules: 47 The following Lewis structures are incorrect. Explain what is wrong with each one and give a correct Lewis structure for the molecule. Incorrect Structure Reason Correct Structure (a) Too many electrons. (b) 48 (c) Hydrogen atoms do not form double bonds. Too few electrons (d) Too many electrons. (e) Fluorine has more than an octet. (f) Oxygen does not have an octet; carbon rarely has a non-bonding electron pair. (g) Too few electrons. The skeletal structure of acetic acid shown below is correct, but some of the bonds are wrong. (a) Identify the incorrect bonds and explain what is wrong with them. (b) Write the correct structure for acetic acid. (a) Neither oxygen atom has a complete octet. The left-most hydrogen atom is forming two bonds (4 e ). Hydrogen can only be surrounded by at most two electrons. − (b) The correct structure is: H H O C C H O H