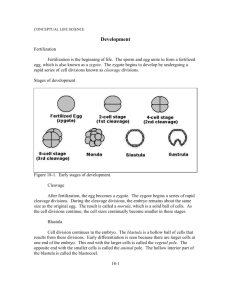
Chapter 44: Animal Development
advertisement

44 Go with the flow P lace your hand over your heart. Now place it over your liver. Next over your appendix. Surely you put your hand first on the left side of your chest, then on your right side just under your ribs, and finally on the right side of your lower abdomen. But in Chapter 31 you learned that vertebrates (including you) are bilaterally symmetrical (left arm/right arm, left kidney/right kidney, and so on). Clearly, however, our bilateral symmetry is not absolute. Some of our internal organs—including the heart, liver, appendix, stomach, and the lobes of the lungs—are oriented differently with respect to the left and right sides of the body. How does a developing embryo know which side is left and which is right? Clues to answering this question came from the fact that in about 1 out of every 7,000 people, the arrangement of the internal organs is reversed, a condition known as situs inversus, Latin for “location inverted.” This developmental difference arises when the very early embryo goes from being a single layer of cells to multiple layers of cells. As you will learn in this chapter, to get from a single layer of cells to the next stage with two layers of cells, a pore or slit forms as cells in one area of the embryo migrate inward from the surface. Other cells from the surface migrate toward and through this opening to take up positions underneath. The place where the inward movement of cells starts is called the node. Cells of the node have motile cilia that sweep extracellular fluid through the opening. Cells bordering the node also have one nonmotile cilium each—a primary cilium. When the primary cilia are bent by the flow of extracellular fluid, they initiate signaling cascades that determine the pattern of internal organ development. Since the fluid driven by the motile cilia tends to flow from right to left, the signaling cascades are not expressed symmetrically, and this initiates the left–right organization of organ development. Among individuals carrying a mutation that eliminates motility of the nodal cilia, half have the normal orientation of the internal organs and half have situs inversus. Most people with this condition lead normal, healthy lives. They may not even know about it Go with the Flow The internal organs of humans are not all symmetrical, and some individuals are born with the mirror-image pattern of what is seen in most people— a condition called situs inversus. The left–right asymmetry of the internal organs is initiated by asymmetrical stimulation of primary cilia at a very early stage in development. This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. CHAPTER OUTLINE 44.1 How Does Fertilization Activate Development? 44.2 How Does Gastrulation Generate Multiple Tissue Layers? 44.3 How Do Organs and Organ Systems Develop? 44.4 How is the Growing Embryo Sustained? 44.5 What Are the Stages of Human Development? 44.1 When Sperm Meet Egg Development begins with the fertilization of an egg by a sperm. Once one sperm fuses with the egg, all other sperm are blocked. An animal egg typically is much larger than the sperm; the egg cytoplasm is loaded with informational molecules and nutrients that will direct development and nourish the growing embryo. unless a routine physical exam reveals that the organs are not where they should be. As frequently happens in biology, we just pushed the question back one more step. Why do the nodal cilia beat in such a way that the extracellular fluid flows from right to left? The root cause of this asymmetry likely originates in some of the early cell divisions of the fertilized egg. The fertilized egg goes through an initial series of cell divisions that subdivide the egg cytoplasm into a mass of undifferentiated cells. Although this mass of cells shows no hints of the eventual body plan, an uneven distribution of molecules from the cytoplasm of the fertilized egg can provide information that directs the fates of cells and sets up the body plan. IN THIS CHAPTER we will see how a single cell becomes a multicellular animal through orderly cell movements that create multiple layers and set up cell–cell interactions. The regional and temporal differences in gene expression that control cell differentiation, described in Chapters 19 and 20, lead to the emergence of the body plan of the animal. We will discuss these early developmental steps in four organisms that have been studied extensively: sea urchins, frogs, chickens, and humans. How Does Fertilization Activate Development? Fertilization is the joining of sperm and egg. You might therefore think of it as the event that begins development. Keep two things in mind, however. First, in animals that reproduce asexually, development proceeds without fertilization. And second, in animals where fertilization does occur, it is preceded by critical events in the maturing egg that will influence subsequent development. Thus, in studying fertilization we are really asking how it activates or restarts multicellular development in sexually reproducing animals. Fertilization does far more than just restore a full diploid complement of maternal and paternal genes. The fusion of sperm and egg plasma membranes accomplishes several things: • It sets up blocks to the entry of additional sperm. • It stimulates ion fluxes across the egg membrane. • It changes the egg’s pH. • It increases egg metabolism and stimulates protein synthesis. • It initiates the rapid series of cell divisions that produce a multicellular embryo. Section 43.2 described the mechanisms of fertilization. Here we take a closer look at the cellular and molecular interactions of sperm and egg that initiate the first steps of development. The sperm and the egg make different contributions to the zygote In most species, eggs are much larger than sperm. Egg cytoplasm is well stocked with organelles, nutrients, and a variety of molecules, including transcription factors and mRNAs. The sperm is little more than a DNA delivery vehicle. Nearly everything the embryo needs during its early stages of development comes from the mother. In addition to providing its haploid nucleus, the sperm makes another important contribution to the zygote in most species—a centriole. The centriole becomes the centrosome of the zygote, which organizes the mitotic spindles for subsequent cell divisions (see Figure 11.10). The centriole is also the origin of the primary cilia of cells, which are important in cell signaling, as we saw in the opening story about situs inversus. Cytoplasmic factors in the egg play important roles in setting up the signaling cascades that orchestrate the major events of development: determination, differentiation, and morphogenesis. This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 924 CHAPTER 44 | ANIMAL DEVELOPMENT Rearrangements of egg cytoplasm set the stage for determination The unique attributes of amphibian eggs make them ideal models for illustrating how rearrangements of egg cytoplasm set the stage for determination. The molecules in the cytoplasm of the amphibian egg are not homogeneously distributed. The entry of the sperm into the egg stimulates rearrangements of the egg cytoplasm that introduce additional organization to the egg cytoplasm. This rearrangement establishes the polarity of the zygote, and when cell divisions begin, the informational molecules that will guide development are not divided equally among daughter cells. Rearrangement of egg cytoplasm following fertilization is easily observed in some frog species because of pigments in the cytoplasm. The nutrients in an unfertilized frog egg are dense yolk granules that are concentrated by gravity in the lower half of the egg, called the vegetal hemisphere. The haploid nucleus of the egg is located at the opposite end, in the animal hemisphere. The outermost (cortical) cytoplasm of the animal hemisphere is heavily pigmented, and the underlying cytoplasm has more diffuse pigmentation. The vegetal hemisphere is not pigmented. Because of these differences, it is easy to observe how the cytoplasm is rearranged when a frog egg is fertilized. The frog egg is radially symmetrical. You can turn it on its vegetal–animal pole axis, and all sides are the same. Spermbinding sites are localized on the surface of the animal hemisphere, so that is where the sperm enters the egg. When a sperm enters the egg, bilateral symmetry is imposed by creating an anterior–posterior axis. Cortical cytoplasm rotates toward the site of sperm entry. This rotation brings different regions of cytoplasm into contact with each other on opposite sides of the egg, producing a band of diffusely pigmented cytoplasm on the side opposite the site of sperm entry. This band, called the gray crescent, marks the location of important developmental events in some species of amphibians (Figure 44.1). The one non-nuclear organelle that the sperm contributes to the egg—the centriole—initiates the cytoplasmic reorganization revealed by the appearance of the gray crescent. The centriole organizes the microtubules in the vegetal hemisphere cytoplasm into a parallel array that guides the movement of the cortical cytoplasm. These microtubules also appear to be directly responsible for movement of specific organelles and proteins, because these organelles and proteins move from the vegetal (A) Fertilization Animal pole Egg b-catenin (orange) is distributed throughout cytoplasm. Sperm GSK-3 (blue), which targets b-catenin for degradation, is also found throughout cytoplasm. Vegetal pole A protein that inhibits GSK-3 is contained in vegetal pole vesicles. (B) Cortical rotation Ventral (V) Dorsal (D) Vesicles in the vegetal pole move on microtubule tracks to the side opposite sperm entry. (C) Dorsal enrichment inhibitor The vesicles release GSK-inhibiting protein… V D (D) Dorsal inhibition of GSK-3 V …so GSK-3 does not degrade b-catenin on the dorsal side… D …but does degrade it on the ventral side. Animal cortical cytoplasm (pigmented) Animal pole (A) The cortical cytoplasm rotates relative to the inner cytoplasm. (E) Dorsal enrichment of β-catenin A Inner cytoplasm Sperm entry point Vegetal pole (V) V Vegetal cortical cytoplasm (unpigmented) V The gray crescent is created by the rotation. 44.1 The Gray Crescent Rearrangement of the cytoplasm of frog eggs after fertilization creates the gray crescent. Thus there is a higher b-catenin concentration in the dorsal cells of the early embryo. D 44.2 Cytoplasmic Factors Set Up Signaling Cascades Cytoplasmic movement changes the distributions of critical developmental signals. In the frog zygote, the interaction of the protein kinase GSK-3, its inhibitor, and the protein β-catenin are crucial in specifying the dorsal–ventral axis of the embryo. This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 44.1 hemisphere to the gray crescent region even faster than the cortical cytoplasm rotates. The movement of cytoplasm, proteins, and organelles changes the distribution of critical developmental signals. A key transcription factor in early development is β-catenin, which is produced from maternal mRNA (mRNA produced and stored in the egg while it was maturing in the ovary). Beta-catenin is found throughout the egg cytoplasm. Also present throughout the egg cytoplasm is a protein kinase called glycogen synthase kinase-3 (GSK-3), which phosphorylates and thereby targets β-catenin for degradation. An inhibitor of GSK-3 is segregated in the vegetal cortex of the egg. After sperm entry, this inhibitor is moved along microtubules to the gray crescent, where it prevents the degradation of β-catenin. As a result, the concentration of β-catenin is higher on the dorsal than on the ventral side of the developing embryo (Figure 44.2). Beta-catenin plays a major role in the cell–cell signaling cascade that begins the process of cell determination and the formation of the embryo. But before cell–cell signaling can occur, multiple cells must be in place. Let’s turn to the early series of cell divisions that transform the zygote into a multicellular embryo. (A) Complete cleavage (frog) | HOW DOES FERTILIZATION ACTIVATE DEVELOPMENT? 925 Cleavage repackages the cytoplasm Transformation of the diploid zygote into a mass of cells occurs through a rapid series of cell divisions called cleavage. Because the cytoplasm of the zygote is not homogeneous, these first cell divisions result in the differential distribution of nutrients and cytoplasmic determinants in the early embryo. In most animals, cleavage proceeds with rapid DNA replication and mitosis but with no cell growth and little gene expression. The embryo becomes a solid ball of smaller and smaller cells. Eventually, this ball forms a central fluid-filled cavity called a blastocoel, at which point the embryo is called a blastula. Its individual cells are called blastomeres. The pattern of cleavage in different species influences the form of their blastulas. • Complete cleavage occurs in most eggs that have little yolk (stored nutrients). In this pattern, early cleavage furrows divide the egg completely and the blastomeres are of similar size. The frog egg undergoes complete cleavage, but because its vegetal pole contains more yolk, the division of the cytoplasm is unequal and the blastomeres in the animal hemisphere are smaller than those in the vegetal hemisphere (Figure 44.3A). Animal pole Vegetal cells have incorporated yolk and are thus larger than the animal cells in the 16-cell embryo. The planes of the second cleavage are displaced only slightly by yolk in the cytoplasm. The embryo forms as a blastodisc that sits on top of the yolk mass. (B) Incomplete cleavage (zebrafish) In birds and fishes, cleavage furrows do not penetrate the large yolk mass. (C) Superficial cleavage (Drosophila) 1 Mitosis (nuclear division) occurs without cell division. 3 The nuclei migrate to the inner edge of the plasma membrane. Nucleus 2 A syncitium—a single cell with many nuclei—is produced. 4 Cellularization occurs, creating a blastoderm 44.3 Some Patterns of Cleavage Differences in patterns of early embryonic development reflect differences in the way the egg cytoplasm is organized. (A) The frog is a model organism representing complete cleavage in these scanning electron micrographs. (B) SEMs of zebrafish embryos illustrate incomplete cleavage, in which the large yolk mass limits the planes of cleavage. (C) Nuclear staining reveals the syncitial nuclei characteristic of the early embryo of a fruit fly. These nuclei migrate to the periphery. Cleavage furrows then move inward to separate the nuclei into individual cells, forming the blastoderm. This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 926 CHAPTER 44 | ANIMAL DEVELOPMENT • Incomplete cleavage occurs in many species in which the egg contains a lot of yolk and the cleavage furrows do not penetrate it all. Discoidal cleavage is a type of incomplete cleavage common in fishes, reptiles, and birds, the eggs of which contain a dense yolk mass. The embryo forms as a disc of cells, called a blastodisc, that sits on top of the yolk mass (Figure 44.3B). • Superficial cleavage is a variation of incomplete cleavage that occurs in insects such as the fruit fly (Drosophila). Early in development, cycles of mitosis occur without cell division, producing a syncytium—a single cell with many nuclei (Figure 44.3C). The nuclei eventually migrate to the periphery of the egg, after which the plasma membrane of the egg grows inward, partitioning the nuclei into individual cells surrounding a core of yolk. The positions of the mitotic spindles during cleavage are not random but are defined by cytoplasmic factors produced from the maternal genome and stored in the egg (see Section 19.4). The orientation of the mitotic spindles can determine the planes of cleavage and the arrangement of the blastomeres. In complete cleavage, if the mitotic spindles of successive cell divisions form parallel or perpendicular to the animal–vegetal axis of the zygote, a pattern of radial cleavage occurs as seen in the frog: the first two cell divisions are parallel to the animal–vegetal axis and the third is perpendicular to it (see Figure 44.3A). Spiral cleavage results when the mitotic spindles are at oblique angles to the animal–vegetal axis. In spiral cleavage, each new cell layer is shifted to the left or right, depending on the orientation of the mitotic spindles. Most mollusks have spiral cleavage, reflected in some species by a coiling shell pattern (as seen in snails). (A) Parallel plane A Plane of first cell division Perpendicular plane Several features of early cell divisions in placental mammals (eutherians) are so different from those seen in other animal groups that some biologists think it is inappropriate to call it cleavage. But whether you call it cleavage or not, it is still the sequence of early cell divisions that produces a body of undifferentiated cells that will become the embryo. This process in mammals is very slow. Cell divisions are 12 to 24 hours apart, compared with tens of minutes to a few hours in non-mammalian species. Also, the cell divisions of mammalian blastomeres are not in synchrony with each other. Because the blastomeres do not undergo mitosis at the same time, the number of cells in the embryo does not increase in the regular (2, 4, 8, 16, 32, etc.) progression typical of other species. The pattern of mammalian cleavage is rotational: the first cell division is parallel to the animal–vegetal axis, yielding two blastomeres. In the second cell division, those two blastomeres divide at right angles to one other: one divides parallel to the animal–vegetal axis, while the other divides perpendicular to it (Figure 44.4A). Another unique feature of the slow, rotational mammalian cleavage is that gene products expressed during cleavage play roles in cleavage. In animals such as sea urchins and frogs, gene transcription does not occur in the blastomeres, and cleavage is therefore directed exclusively by molecules that were present in the egg before fertilization. As in other animals that have complete cleavage, the early cell divisions in a mammalian zygote produce a loosely associated ball of cells. After the 8-cell stage, however, the behavior of the mammalian blastomeres changes. They change shape to maximize their surface contact with one another, form tight junctions, and become a compact mass of cells (Figure 44.4B). At the transition from the 16- to the 32-cell stage (the fourth division), the cells separate into two groups. The inner cell mass will become the embryo, while the surrounding outer cells become an encompassing sac called the trophoblast. Trophoblast cells secrete fluid, creating a cavity—the blastocoel—with the in- 44.4 Becoming a Blastocyst (A) Mammals have rotational cleavage, in which the plane of the first cleavage is parallel to the animal–vegetal (A–V) axis, but the second cell division involves two planes (beige) at right angles to each other. (B) Scanning electron micrographs show that asynchronous cell division results in an asymmetrical blastocyst at about the 32-cell stage. (C) Seen in cross section under a light microscope, the mammalian blastocyst consists of an inner cell mass adjacent to a fluid-filled blastocoel and surrounded by trophoblast cells. V (B) 8-cell stage Early cell divisions in mammals are unique (C) 16-cell stage 32-cell stage Blastocyst (cross section) Trophoblast (outer cells) Blastocoel The inner cell mass will form the embryo. This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 44.1 16–32 cells (3–4 days postfertilization) Implantation of blastocyst (6–7 days postfertilization) Ovary 2–4 cells (2 days postfertilization) Site of fertilization Uterus Cervix Vagina Human embryo at 9 days Wall of uterus Developing placenta Inner cell mass (embryo) Amnion Hypoblast Emerging chorionic villus Epiblast Trophoblast Blastocoel Blood vessel Endometrium | HOW DOES FERTILIZATION ACTIVATE DEVELOPMENT? 927 44.5 A Human Blastocyst at Implantation Adhesion molecules and proteolytic enzymes secreted by trophoblast cells allow the blastocyst to burrow into the endometrium. Once the blastocyst is implanted in the wall of the uterus, the trophoblast cells send out numerous projections—the chorionic villi—which increase the embryo’s area of contact with the mother’s bloodstream. bryo, a connection develops between the circulatory systems of the embryo and the mother. As we will see later in this chapter, the structures that provide this connection are the placenta and the umbilical cord. Thus, the mammalian blastocyst must produce both the embryo (from the inner cell mass) and its support structures (from the trophoblast). Fertilization in mammals occurs in the upper reaches of the mother’s oviduct, and cleavage occurs as the zygote travels down the oviduct to the uterus. When the blastocyst arrives in the uterus, the trophoblast adheres to the lining of the uterus (the endometrium), beginning the process of implantation. In humans, implantation begins about 6 days after fertilization and is aided by adhesion molecules and enzymes secreted by the trophoblast (Figure 44.5). As the blastocyst moves down the oviduct to the uterus, it must not embed itself in the oviduct (Fallopian tube) wall, or the result will be an ectopic, or tubal, pregnancy—a very dangerous condition. Early implantation is prevented by the zona pellucida, which surrounded the egg and remains around the cleaving ball of cells (see Section 43.2). At about the time the blastocyst reaches the uterus, it hatches from the zona pellucida, and implantation can occur. Specific blastomeres generate specific tissues and organs ner cell mass at one end (Figure 44.4C). At this stage, the mammalian embryo is called a blastocyst, distinguishing it from the blastulas of other animal groups. Why is mammalian cleavage so different? A key factor is that mammalian eggs contain no yolk and must derive all nutrients from the mother. Mammals are viviparous: the embryo develops within the uterus of the mother. To support the developing em- Animal pole Ectoderm will form epidermal layer of skin. The neural ectoderm (midline) will form the nervous system. The gray crescent is the site where major cell movement will begin. (See Figure 44.1) Endoderm will form the lining of the gut, the liver, and the lungs. Vegetal pole Mesoderm will form muscle, bone, kidneys, blood, gonads, and connective tissues. Cleavage results in a repackaging of the egg cytoplasm into a large number of small cells surrounding the fluid-filled blastocoel. Except in mammals, little cell differentiation and little if any gene expression occur during cleavage. Nevertheless, cells in different regions of the blastula possess different complements of the nutrients and cytoplasmic determinants that were present in the egg. The blastocoel prevents cells from different regions of the blastula from coming into contact and interacting, but that will soon change. During the next stage of development, the cells of the blastula will move around and come into new associations with one another, communicate instructions to one another, and begin to differentiate. In many animals, these movements of the blastomeres are so regular and well orchestrated that it is possible to label a specific blastomere with a dye and identify the tissues and organs that form from its progeny. Such labeling experiments produce fate maps of the blastula (Figure 44.6). Blastomeres become determined—committed to specific fates—at different times in different species. In some species, 44.6 Fate Map of a Frog Blastula Colors indicate the portions of the blastula that will form the three germ layers and subsequently the frog’s tissues and organs. This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 928 CHAPTER 44 | ANIMAL DEVELOPMENT such as roundworms, the fates of blastomeres are restricted as early as the two-cell stage. If one of these blastomeres is experimentally removed, a particular portion of the embryo will not form. This type of development has been called mosaic development because each blastomere seems to contribute a specific set of “tiles” to the final “mosaic” that is the adult animal. In contrast to mosaic development, the loss of some cells during cleavage in regulative development does not affect the developing embryo, because the remaining cells compensate for the loss. Regulative development is typical of many vertebrate species, including humans. The pluripotent cells of the mammalian blastocyst (the inner cell mass) are known as embryonic stem cells and are the subject of much research, particularly because of their therapeutic potential (see Section 19.2). If some blastomeres can change their fate to compensate for the loss of other cells during cleavage and blastula formation, can those cells form an entire embryo? To a certain extent, yes. During cleavage or early blastula formation in mammals, for example, if the blastomeres are physically separated into two groups, both groups can produce complete embryos. Since the two embryos come from the same zygote, they will be monozygotic twins—genetically identical. Non-identical twins occur when two separate eggs are fertilized by two separate sperm. Thus, while identical twins are always of the same sex, non-identical twins have a 50 percent chance of being the same sex. In about 1 out of 50,000 human pregnancies, genetic or environmental factors cause the inner cell mass to split partially. The result is twins that are conjoined at some point on their bodies, usually sharing some of their organs and limbs. 44.1 RECAP The egg is stocked with nutrients and informational molecules that power and direct the early stages of development. Fertilization activates the egg and stimulates rearrangement of the cytoplasm, setting up the body axes and positional information that initiate signaling cascades, which control determination and differentiation. • Explain how β-catenin becomes concentrated in only certain blastomeres. See p. 925 and Figure 44.2 • In general terms, describe the difference between complete and incomplete cleavage. See pp. 925–926 and Figure 44.3 • What does a fate map tell us? How are fate maps constructed? See pp. 927–928 and Figure 44.6 Of the next stage of development—gastrulation—the developmental biologist Louis Wolpert once said, “It is not birth, marriage, or death, but gastrulation which is the most important time in your life.” During gastrulation, cell movements create new cell-to-cell contacts, which in turn sets up signaling cascades. Signaling cascades initiate the differentiation of cells and tissues and set the stage for the emergence of the body plan. 44.2 How Does Gastrulation Generate Multiple Tissue Layers? The blastula is typically a fluid-filled ball of cells. How does this simple ball of cells become an embryo made up of multiple tissue layers with head and tail ends and dorsal and ventral sides? Gastrulation is the process whereby the blastula is transformed by massive movements of cells into an embryo with multiple tissue layers and distinct body axes. The resulting spatial relationships between tissues make possible the inductive interactions between cells that trigger differentiation and organ formation (see Figure 19.10). During gastrulation, three germ layers (also called cell layers or tissue layers) form (see Figure 44.6): • The endoderm is the innermost germ layer, created as some blastomeres move to the inside of the embryo. The endoderm gives rise to the lining of the digestive tract, respiratory tract, pancreas, and liver. • The ectoderm is the outer germ layer, formed from those cells remaining on the outside of the embryo. The ectoderm gives rise to the nervous system, including the eyes and ears; and to the epidermal layer of the skin and structures derived from skin, such as hair, feathers, nails or claws, sweat glands, oil glands, and even teeth and other tissues of the mouth. • The mesoderm is the middle layer and is made up of cells that migrate between the endoderm and the ectoderm. The mesoderm contributes tissues to many organs, including the heart, blood vessels, muscles, and bones. Some of the most interesting and important challenges in animal development have dealt with two related questions: what directs the cell movements of gastrulation, and what is responsible for the resulting patterns of cell differentiation and organ formation? Scientists have made significant progress in answering both these questions at the molecular level. In the following discussion, we will begin with sea urchin gastrulation because it is the simplest to conceptualize in spatial terms. We will then describe the more complex pattern of gastrulation in frogs, which in turn will help elucidate the still more complex patterns in reptiles, birds, and mammals. Invagination of the vegetal pole characterizes gastrulation in the sea urchin The sea urchin blastula is a hollow ball of cells only one cell thick. The end of the blastula stage is marked by slowing of the rate of mitosis; the beginning of gastrulation is marked by a flattening of the vegetal hemisphere (Figure 44.7). Some cells at the vegetal pole bulge into the blastocoel, break away from neighboring cells, and migrate into the cavity. These cells become mesenchyme —cells of the middle germ layer, the mesoderm. Mesenchymal cells are not organized in tightly packed sheets or tubes like epithelial cells are; they act as independent units, migrating into and among the other tissue layers. The flattening at the vegetal pole results from changes in the shape of individual blastomeres. These cells, which are origi- This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 44.2 1 The vegetal pole of the blastula flattens. 2 Some cells change shape and move inward to form the archenteron. | HOW DOES GASTRULATION GENERATE MULTIPLE TISSUE LAYERS? 3 Other cells break free, becoming primary mesenchyme. Animal hemisphere 4 More cells break free, forming secondary mesenchyme. Thin extensions of these cells (filopodia) attach to the overlying ectoderm. 5 The archenteron is 929 6 The mouth will elongated by contraction of mesenchymal filopodia and cell rearrangement. form where the archenteron meets ectoderm. Secondary mesenchyme Ectoderm Endoderm Archenteron Vegetal hemisphere Primary mesenchyme Blastopore 7 The blastopore will form the 44.7 Gastrulation in Sea Urchins During gastrulation, cells move to new positions and form the three germ layers from which differentiated tissues develop. anus of the mature animal. yo u r B i oPort al.com GO TO Animated Tutorial 44.1 • Gastrulation nally rather cuboidal, become wedge-shaped, with smaller outer edges and larger inner edges. As a result, the vegetal pole bulges inward, or invaginates, as if someone were poking a finger into a hollow ball (see Figure 44.7). The invaginating cells become endoderm and form the primitive gut, called the archenteron. At the tip of the archenteron, more cells enter the blastocoel to form more mesoderm. Changes in cell shapes cause the initial invagination of the archenteron, but eventually it is pulled by the mesenchyme cells. These cells, attached to the tip of the archenteron, send out extensions called filopodia that adhere to the overlying ectoderm. When the filopodia contract, they pull the archenteron toward the ectoderm at the opposite end of the embryo from where the invagination began. The mouth of the animal forms where the archenteron makes contact with this overlying ectoderm. The opening created by the invagination of the vegetal pole is called the blastopore; it will become the anus of the animal. What mechanisms control the various cell movements of sea urchin gastrulation? The immediate answer is that specific properties of particular blastomeres change. For example, some vegetal cells change shape and bulge into the blastocoel, and these cells become mesenchyme. Once they lose contact with their neighboring cells on the surface of the blastula, they send out filopodia that then move along an extracellular matrix of proteins laid down by the cells lining the blastocoel. A deeper understanding of gastrulation requires that we discover the molecular mechanisms whereby different blastomeres develop different properties. Cleavage systematically divides up the cytoplasm of the egg. The sea urchin blastula at the 64cell stage is radially symmetrical, but it has polarity, as described in Section 19.4. It consists of tiers of cells. As in the frog blastula, the top is the animal pole and the bottom the vegetal pole. If different tiers of blastula cells are separated, they show different developmental potentials; only cells from the vegetal pole are capable of initiating the development of a complete larva (see Figure 19.8). It has been proposed that these differences are due to uneven distribution of various transcriptional regulatory proteins in the egg cytoplasm. As cleavage progresses, these proteins end up in different groups of cells. Therefore, specific sets of genes are activated in different cells, determining their different developmental capacities. Let’s turn now to gastrulation in the frog, in which several key signaling molecules have been identified. Gastrulation in the frog begins at the gray crescent Amphibian blastulas have considerable yolk and are more than one cell thick; therefore, gastrulation is more complex in amphibians than in sea urchins. Variation is considerable among different species of amphibians, but in this brief account we will use results from studies done on different species to produce a generalized picture of amphibian development. Amphibian gastrulation begins when certain cells in the gray crescent region change their shapes and cell adhesion properties. These cells bulge inward toward the blastocoel while they remain attached to the outer surface of the blastula by slender necks. Because of their shape, these cells are called bottle cells. Bottle cells mark the spot where the dorsal lip of the blastopore will form (Figure 44.8). As the bottle cells move inward, the dorsal lip is created, and a sheet of cells moves over it into the blastocoel. This process is called involution. One group of involuting cells is the prospective endoderm; these cells form the primitive gut, or archenteron. Another group will move between the endoderm and the outermost cells to form the mesoderm. These rearrangements are due to changes in cell properties called convergent extension. The cells elongate in the direction of movement, but they also intercalate (move in between each other). If they just elongated, the migrating group of cells would become much narrower; by intercalating, they maintain the width of the migrating cell group. As gastrulation proceeds, cells from the animal hemisphere flatten and move toward the site of involution in a process called epiboly. The blastopore lip widens and eventually forms a com- This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 930 CHAPTER 44 | ANIMAL DEVELOPMENT Animal pole Blastocoel 1 Gastrulation begins when cells in the region of the gray crescent move inward, forming the dorsal lip of the future blastopore. Bottle cells Dorsal lip of blastopore Vegetal pole Blastocoel Bottle cells Archenteron begins to form 2 Cells of the animal Dorsal lip pole spread out, pushing surface cells below them toward and across the dorsal lip. These cells involute into the interior of the embryo, where they form the endoderm and mesoderm. Archenteron Mesoderm Dorsal lip Blastocoel displaced by mesoderm Endoderm Archenteron (future digestive tract) 3 Involution creates the archenteron and destroys the blastocoel. The blastopore lip forms a circle, with cells moving to the interior all around the blastopore; the yolk plug is visible through the blastopore. a dorsal–ventral and anterior–posterior organization. Most importantly, the fates of specific regions of the endoderm, mesoderm, and ectoderm have been determined. The beautiful experiments revealing how determination takes place in the amphibian embryo are an old but exciting story. Ectoderm Mesoderm (notochord) Dorsal lip Yolk plug Ventral lip of blastopore 44.8 Gastrulation in the Frog Embryo The colors in this diagram are matched to those in Figure 44.6, the frog fate map. plete circle surrounding a “plug” of yolk-rich cells. As cells continue to move inward through the blastopore, the archenteron grows, gradually displacing the blastocoel. As gastrulation comes to an end, the amphibian embryo consists of three germ layers: ectoderm on the outside, endoderm on the inside, and mesoderm in between. The embryo also has The dorsal lip of the blastopore organizes embryo formation In the early 1900s, the German biologist Hans Spemann was studying the development of salamander eggs. He was interested in finding out whether the nuclei of blastomeres remain capable of directing the development of complete embryos. With great patience and dexterity, he formed loops from single hairs taken from a baby (in fact, his daughter) and tied them around fertilized eggs along the plane of the first cell division, effectively dividing the eggs in half, with the nucleus restricted to one side. That side went through cell divisions and developed into a salamander; the other half simply degenerated. Up until the 16-cell stage, if one nucleus escaped to the other side of the constriction, twin salamanders could develop. Thus, each of the nuclei of the blastula (at least up to the 16-cell stage) was capable of directing and supporting development of the whole organism. But, as often happens in science, Spemann’s bisection experiments revealed a new phenomenon. Sometimes the half of the blastula receiving an escaped nucleus did not develop. When his loops bisected the gray crescent, both halves of the zygote developed into a complete embryo. When he tied the loops so the gray crescent was on only one side of the constriction, however, only that half of the zygote developed into a complete embryo (Figure 44.9). The half lacking gray crescent material underwent cell division, but even if it contained a nucleus, it became a clump of undifferentiated cells that Spemann called a “belly piece.” Spemann hypothesized that cytoplasmic factors unequally distributed in the fertilized egg were necessary for gastrulation and the development of a normal salamander. To further test the hypothesis that cells receiving different complements of cytoplasmic factors had different developmental fates, Spemann transplanted pieces of early gastrulas to various locations on other gastrulas. Guided by fate maps (see Figure 44.6), he was able to take a piece of ectoderm he knew would develop into skin and transplant it to a region that normally becomes part of the nervous system, and vice versa. When he performed these transplants in early gastrulas— when the blastopore was just beginning to form—the transplanted pieces always developed into tissues that were appropriate for the location where they were placed. Transplanted cells destined to become epidermis in their original location developed into nervous system tissue, and transplanted cells destined to become nervous system tissue in their original location developed into host epidermis. Thus, Spemann learned that the fates of the transplanted cells had not been determined before the transplantation (see Figure 19.2). In late gastrulas, however, the same experiment yielded opposite results. Transplanted cells destined to become epidermis in their original location produced patches of skin cells in the host This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 44.2 Gray crescent bisected | HOW DOES GASTRULATION GENERATE MULTIPLE TISSUE LAYERS? Gray crescent isolated 931 INVESTIGATING LIFE 44.10 The Dorsal Lip Induces Embryonic Organization 1 Using a baby’s hair, In a classic experiment, Hans Spemann and Hilde Mangold transplanted the dorsal blastopore lip mesoderm of an early gastrula stage salamander embryo. The results showed that the cells of this embryonic region, which they dubbed “the organizer,” could direct the formation of an entire embryo. Spemann constricted a salamander zygote along the plane of first cleavage. HYPOTHESIS Cytoplasmic factors in the early dorsal blastopore lip 2a This constriction bisects organize cell differentiation in amphibian embryos. the gray crescent. 2b This constriction METHOD restricts the gray crescent to one half of the zygote. 1. Excise a patch of mesoderm tissue from above the dorsal blastopore lip of an early gastrula stage salamander embryo (the donor). 2. Transplant the donor tissue onto a recipient embryo at the same stage. The donor tissue is transplanted onto a region of ectoderm that should become epidermis (skin). Gray crescent Blastocoel 3 Only those halves Presumptive mesoderm with gray crescent develop normally. “Belly piece” Normal Normal Normal Dorsal blastopore lip 44.9 Gastrulation and the Gray Crescent Spemann’s research revealed that gastrulation and subsequent normal development in salamanders depends on cytoplasmic determinants localized in the gray crescent. RESULTS nervous system, and the transplanted cells from regions that would develop into nervous system tissue produced neural tissue in the skin of the recipient. At some point during gastrulation, the fates of the embryonic cells had become determined. Spemann’s next experiment, done with his student Hilde Mangold, produced momentous results: they transplanted the dorsal lip of the blastopore (Figure 44.10). When this small piece of tissue was transplanted into the presumptive belly area of another gastrula, it stimulated a second site of gastrulation—and a second complete embryo formed belly-to-belly with the original embryo. Because the dorsal lip of the blastopore was apparently capable of inducing the host tissue to form an entire embryo, Spemann and Mangold dubbed the dorsal lip tissue the primary embryonic organizer, or simply the organizer. For more than 80 years, the organizer has been an active area of research. yo u r B i oPort al.com GO TO Animated Tutorial 44.2 • Tissue Transplants Reveal the Process of Determination Transcription factors underlie the organizer’s actions Primary involution (recipient of dorsal lip) C Presumptive epidermis 2 … and a second set of dorsal Induced nervous system neural structures forms in the recipient embryo. Mesoderm Nervous system 1 The donor tissue induces a Endoderm secondary involution… 3 Eventually a complete secondary embryo forms, attached to the original embryo at the belly. CONCLUSION The cells of the dorsal blastopore lip can induce other cells to change their developmental fates. Go to yourBioPortal.com for original citations, discussions, and relevant links for all INVESTIGATING LIFE figures. With the advent of modern molecular methods, the primary embryonic organizer has been studied intensively to discover the molecular mechanisms involved This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 932 CHAPTER 44 | ANIMAL DEVELOPMENT in its action. The distribution of the transcription factor β-catenin in the late blastula corresponds to the location of the organizer in the early gastrula, so β-catenin is a candidate for the initiator of organizer activity. To prove that a protein is an inductive signal, it has to be shown that it is both necessary and sufficient for the proposed effect. In other words, the effect should not occur if the candidate protein is not present (necessity), and the candidate protein should be capable of inducing the effect where it would otherwise not occur (sufficiency). The criteria of necessity and sufficiency have been satisfied for β-catenin. If β-catenin mRNA transcripts are depleted by injections of antisense RNA into the egg (see Section 18.4), gastrulation does not occur. If β-catenin is experimentally overexpressed in another region of the blastula, it can induce a second axis of embryo formation, as the transplanted dorsal lip did in the Spemann–Mangold experiments. Thus, β-catenin appears to be both necessary and sufficient for the formation of the primary embryonic organizer—but it is only one component of a complex signaling process. How the presence of β-catenin creates the organizer, and how the organizer then induces the beginnings of the body plan, involves a complex series of interactions between transcription factors and growth factors that control gene expression. What follows is only a portion of this complex and still emerging story. What you should take from this description is not the names of the genes and gene products involved. Rather, we hope you will gain a basic appreciation for how signaling molecules interact to produce different combinations of signals that convey positional and temporal information. This information guides cells into different paths of determination and differentiation. Studies of early gastrulas revealed that primary embryonic organizer activity is generated by the interaction of β-catenin with signals coming from the vegetal cells. Together, they activate the expression of the transcription factor Goosecoid. Expression of the goosecoid gene depends on two signaling pathways. The first of these pathways involves a goosecoid-promoting transcription factor called Siamois. The siamois gene is normally repressed by a ubiquitous transcription factor called Tcf-3, but in cells in which β-catenin is present, an interaction between Tcf3 and β-catenin induces siamois expression (Figure 44.11). But Siamois protein alone is not sufficient for goosecoid expression. The second pathway involves mRNAs from the original egg cytoplasm for a family of proteins called transforming growth factor-β (TGF-β). TGF-β interacts with the Siamois protein to control goosecoid transcription. Thus, you can see that it is a complex combination of factors that determines which cells become the primary organizer. The organizer changes its activity as it migrates from the dorsal lip Organizer cells begin the process of formation of the dorsal lip of the blastopore. Specifically, these cells are at the center of the dorsal lip and involute, moving forward on the midline (i.e., the middle of the anterior–posterior axis). The first organizer cells to enter the embryo migrate anteriorly to become the head endoderm and head mesoderm. Here, they induce neighboring Gray crescent 1 Repression of siamois by Tcf-3 proteins prevents expression of organizer-specific genes. 2 b-Catenin in vegetal cells below the gray crescent blocks Tcf-3 repression of siamois gene expression. No b-catenin Tcf-3 proteins siamois gene repressed DNA b-catenin proteins No transcription siamois gene activated Transcription Siamois protein 3 TGF-b-related signaling pathway acts synergistically with Siamois to activate the goosecoid gene. 4 Goosecoid protein goosecoid Transcription activates numerous genes in the organizer. 44.11 Molecular Mechanisms of the Organizer In amphibians, the organizing potential of the gray crescent depends on the activity of the goosecoid gene, which in turn is activated by signaling pathways set up in the vegetal cells below the gray crescent. cells to participate in making structures of the head. Later organizer cells that involute into the embryo will induce structures of the trunk, and the last of the organizer cells to move inward from the dorsal lip will induce structures of the tail. How does the nature of the organizer cells change to enable them to induce head, trunk, or tail structures? Inductive tissue interactions can suppress as well as activate. As we learned above, the early organizer cells express the transcription factor Goosecoid, which activates genes encoding soluble signals. As these cells move forward in the blastocoel, they come into contact with new populations of cells that produce a number of different growth factors. For head structures to form, certain of these growth factors have to be suppressed. The anteriormost organizer cells, under the influence of Goosecoid, produce and release antagonists to those growth factors. The induction of trunk structures requires suppression of a different set of growth factors. In organizer cells that involute later than the head organizers, Goosecoid is no longer the dominant transcription factor, and these cells express different growth factor antagonists. The induction of tail structures requires still different activities of the organizer cells that involute last. Thus, the organizer cells express appropriate sets of growth factor antagonists at the right times to achieve different patterns of differentiation on the anterior–posterior axis. The initiation of the development of the nervous system also involves a suppressive tissue interaction. For a long time it was thought that the involuting organizer cells actively induced the This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 44.2 | HOW DOES GASTRULATION GENERATE MULTIPLE TISSUE LAYERS? INVESTIGATING LIFE 44.12 Differentiation Can Be Due to Inhibition of Transcription Factors When organizer cells involute to underlie dorsal ectoderm along the embryo midline, that overlying ectoderm becomes neural tissue rather than skin (epidermis). But do the organizer cells cause dorsal ectoderm to become neural tissue, or do they prevent this ectoderm from becoming skin? HYPOTHESIS The default state of amphibian dorsal ectoderm is neural; it is induced by underlying mesoderm to become epidermis. METHOD 1. Excise the animal caps of late-stage frog blastulas and disperse the cells in culture medium so there is no cell-to-cell contact. From the culture, extract molecules of BMP4 (secreted by mesoderm cells) and molecules of an inhibitor of BMP4. Blastula Dispersed animal cap cells in culture Animal cap Gray crescent 2. Prepare four separate cultures of embryonic ectodermal cells. Incubate with no additions (control); with BMP4 from step 1; with BMP4 inhibitor from step 1; and with both molecules. Control Add BMP4 Add inhibitor of BMP4 Add BMP4 + inhibitor Incubate 3. After incubation, extract mRNAs from the ectodermal cells and analyze for the presence of mRNAs for marker proteins NCAM (neural cell adhesion molecule, a neural protein) and/or keratin (an epidermal protein). RESULTS The control ectoderm (no inductive factors added) expresses the neural marker. In the presence of mesodermal BMP4, ectoderm expresses the epidermal marker. If BMP4 is inhibited, ectoderm expresses the neural marker. BMP4 BMP4 + Control BMP4 inhibitor inhibitor This control message is from a gene expressed in all cells and verifies that each sample contains similar amounts of mRNA. Marker proteins NCAM Keratin “Loading control” CONCLUSION The default state of amphibian dorsal ectoderm is neural. BMP4 protein from mesoderm can induce ecotoderm cells to differentiate into epidermis. Thus the organizer cells must secrete an inhibitor of BMP4. Go to yourBioPortal.com for original citations, discussions, and relevant links for all INVESTIGATING LIFE figures. 933 overlying ectoderm to form neural tissue rather than becoming epidermis. We now know, however, that epidermis is not the default state of the dorsal ectoderm. Rather, the underlying mesoderm secretes factors called BMP proteins that induce the ectoderm to become epidermis. The role of the involuting organizer cells is to block that induction, allowing the overlying ectodermal cells to follow what is really their default pathway—differentiation into neural tissue (Figure 44.12). Reptilian and avian gastrulation is an adaptation to yolky eggs The eggs of reptiles and birds contain a mass of yolk, and the blastulas of these groups develop as a disc of cells on top of the yolk (see Figure 44.3B). We will use the chicken egg to show how gastrulation proceeds in a flat disc of cells rather than in a ball of cells. Cleavage in the chick results in a flat, circular layer of cells called a blastodisc (Figure 44.13). Between the blastodisc and the yolk mass is a fluid-filled space. Some cells from the blastodisc break free and move into this space. These cells come together to form a continuous layer called the hypoblast, which will later contribute to extraembryonic membranes that will support and nourish the developing embryo. The overlying cells make up the epiblast, from which the embryo proper will form. Thus, the avian blastula is a flattened structure consisting of an upper epiblast and a lower hypoblast, which are joined at the margins of the blastodisc. The blastocoel is the fluid-filled space between the epiblast and hypoblast. Gastrulation begins with a thickening in the posterior region of the epiblast, caused by the movement of cells toward the midline and then forward along the midline (see Figure 44.13). The result is a midline ridge called the primitive streak. A depression called the primitive groove forms along the length of the primitive streak. The primitive groove functions as the blastopore, and cells migrate through it into the blastocoel to become endoderm and mesoderm. In the chick embryo, no archenteron forms, but the endoderm and mesoderm migrate forward to form the gut and other structures. At the anterior end of the primitive groove is a thickening called Hensen’s node, which in birds, reptiles, and mammals is the equivalent of the dorsal lip of the amphibian blastopore. Many signaling molecules that have been identified in the frog organizer are also expressed in Hensen’s node. Some cells that pass over This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 934 CHAPTER 44 | ANIMAL DEVELOPMENT 44.13 Gastrulation in Birds Because their eggs contain a large yolk mass, bird and reptile embryos have a flattened blastodisc and display a pattern of gastrulation very different from that of amphibians. Chick embryo viewed from above Flattened blastodisc Yolk 4 …forming the primitive groove— 1 Posterior epiblast cells change shape and thicken, forming the primitive streak. 2 Cells migrate, converging at the primitive streak and causing it to elongate. 5 Cells generated in Hensen’s the chick blastopore. Cells ingress to the embryo interior through Hensen’s node at the anterior end of the groove. 3 The primitive streak narrows and lengthens… node and passing into the gastrula migrate anteriorly and form head structures and notochord. Hensen’s node Anterior Midline Embryo Yolk Posterior Primitive streak Hensen’s node Surface cells move toward the groove and into the gastrula. Hensen’s node become the notochord and organize the chick embryo in a manner similar to that of the frog embryo. And, as we learned at the start of this chapter, the asymmetrical flow of extracellular fluid over this node stimulates the primary cilia of nodal cells, creating asymmetrical signaling cascades that determine the left–right asymmetry of the internal organs. Primitive groove Hensen’s node Cells moving over the sides of the primitive groove form mesoderm and endoderm. Epiblast Endoderm The hypoblast is displaced by spreading endoderm. Blastocoel Yolk Hypoblast Placental mammals retain the avian– reptilian gastrulation pattern but lack yolk Mammalian embryos (with the exception of monotremes) derive their nourishment from the maternal circulation, and therefore mammalian eggs do not have large amounts of yolk to constrain their patterns of cleavage and early development. Nevertheless, mammals and birds evolved from reptilian ancestors, so it is not surprising that they share certain patterns of early development. Earlier we described the development of the mammalian inner cell mass (the equivalent of the avian epiblast) and the outer trophoblast. As in avian development, in placental mammals the inner cell mass splits into an upper layer called the epiblast and a lower layer called the hypoblast. The embryo forms from the epiblast, while the hypoblast contributes to the extraembryonic membranes that will encase the developing embryo and help form the placenta (see Figure 44.5). The epiblast also contributes to the extraembryonic membranes; specifically, it splits off an upper layer of cells that will form the amnion. The amnion will grow to surround the developing embryo as a membranous sac filled with amniotic fluid. Gastrulation occurs in the mammalian epiblast just as it does in the avian epiblast. A primitive groove forms, and epiblast cells migrate through the groove to become layers of endoderm and mesoderm. Primitive groove Cross section through chick embryo 44.2 RECAP The cell movements of gastrulation convert the blastula into an embryo with three tissue layers. New contacts between cells set up inductive signaling interactions that determine cell fates. Dorsal lip tissue is the source of organizer cells that induce development of preliminary head, trunk, and tail structures. • Describe and compare the cell movements that occur during gastrulation in a sea urchin, a frog, and a bird. See Figures 44.7, 44.8, and 44.13 • Explain the molecular basis for the inductive capabilities of the organizer. See pp. 931–932 and Figures 44.11 and 44.12 We have described how the fertilized egg develops into an embryo with three germ layers and how cellular signals trigger different patterns of differentiation. In the next section we will describe how organs and organ systems develop. This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 44.3 44.3 How Do Organs and Organ Systems Develop? Gastrulation produces an embryo with three germ layers that are positioned to influence one another through inductive tissue interactions. During the next phase of development, called organogenesis, many organs and organ systems develop simultaneously and in coordination with one another. An early process of organogenesis in chordates that is directly related to gastrulation is neurulation. Neurulation is the initiation of the nervous system. We will examine neurulation in the amphibian embryo, but it occurs in a similar fashion in reptiles, birds, and mammals. | HOW DO ORGANS AND ORGAN SYSTEMS DEVELOP? 935 Neural plate (A) (B) Neural fold Neural groove Notochord Ectoderm Gut Mesoderm Endoderm (C) Neural tube The stage is set by the dorsal lip of the blastopore Notochord As we learned in the previous section, one group of cells that passes over the dorsal lip of the blastopore moves anteriorly and becomes the endodermal lining of the digestive tract. The other group of cells that involutes over the dorsal lip becomes chordamesoderm, so named because it forms a rod of mesoderm—the notochord—that extends down the center of the embryo. These cells also have important organizer functions (see Figure 44.8). The notochord gives structural support to the developing embryo and is eventually replaced by the vertebral column. The organizing capacity of the chordamesoderm enables the overlying ectoderm to become neural ectoderm (see Figure 44.12). It does this by expressing signaling molecules (one appropriately called Noggin and another one called Chordin) that initiate differentiation of the different divisions of the nervous system. Neurulation involves the formation of an internal neural tube from an external sheet of cells. The first signs of neurulation are flattening and thickening of the ectoderm overlying the notochord; this thickened area forms the neural plate (Figure 44.14A). The edges of the neural plate that run in an anterior–posterior direction continue to thicken to form ridges or folds. Between these neural folds, a groove forms and deepens as the folds roll over it to converge on the midline. The folds fuse, forming a cylinder, the neural tube, and a continuous overlying layer of epidermal ectoderm (Figure 44.14B–D). Cells from the most lateral portions of the neural plate do not become part of the neural tube, but disassociate from it and come to lie between the neural tube and the overlying epidermis. These neural crest cells migrate outward to lead the development of the connections between the central nervous system (brain and spinal cord) and the rest of the body. The neural tube develops bulges at the anterior end, which become the major divisions of the brain; the rest of the tube becomes the spinal cord. In humans, failure of the neural folds to fuse in this posterior region results in a birth defect known as spina bifida. If the folds fail to fuse at the anterior end, an infant can develop without a forebrain—a condition called anencephaly. Although several genetic factors can cause these defects, other factors are environmental, including maternal diet. The incidence of neural tube defects in the United States in the early 1900s was as high as 1 in 300 live births; today it is less than 1 Coelom Epidermis Mesoderm Neural tube Neural crest cells Gut (D) Neural tube Somite Notochord Epidermis Coelom 44.14 Neurulation in a Vertebrate (A) At the start of neurulation, the ectoderm of the neural plate (green) is flat. (B) The neural plate invaginates and folds, forming a tube. (C,D) The completely formed neural tube seen in (C) diagrammatic form and (D) in a scanning electron micrograph of a chick embryo. in 1,000. A major factor in this improvement has been the inclusion of folic acid (a B vitamin, also known as folate) in the mother’s diet. It is essential for pregnant women to ingest sufficient folic acid. Body segmentation develops during neurulation The vertebrate body plan, like that of arthropods, consists of repeating segments that are modified during development. These segments are most evident as the repeating patterns of vertebrae, ribs, nerves, and muscles along the anterior–posterior axis. As the neural tube forms, mesodermal tissues gather along the sides of the notochord to form separate, segmented blocks This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 936 CHAPTER 44 | ANIMAL DEVELOPMENT (A) (B) Neural tube Somites 2-Day chick embryo Neural crest Epidermis Somites Neural tube Notochord 4-Day chick embryo Neural crest cells Neural tube 1 Repeating segments of tissue–somites– form from mesoderm on either side of the neural tube. 2 Each somite divides into three layers of cells. The upper will contribute to skin… muscles… Migrating mesenchyme cells Mesodermal tissue (will become somites) 3 …the middle to Somite forming 4 …and the lower mesenchyme will form cartilage of the vertebrae and ribs. 7-Day chick embryo 5 Neural crest cells migrate between the layers and will produce nerves and other tissue. of cells called somites (Figure 44.15). The somites produce cells that will become the vertebrae, ribs, muscles of the trunk and limbs, and the lower layer of the skin. Nerves that connect the brain and spinal cord with tissues and organs throughout the body are also arranged segmentally. The somites help guide the organization of these peripheral nerves, but the nerves are not of mesodermal origin. As we saw above, when the neural tube fuses, the neural crest cells break loose and migrate inward between the epidermis and the somites and through the somites. These neural crest cells have diverse fates, including the development of peripheral nerves. As development progresses, the different segments of the body change. Regions of the spinal cord differ, regions of the vertebral column differ in that some vertebrae grow ribs of various sizes and others do not, forelegs arise in the anterior part of the embryo, and hind legs arise in the posterior region. Hox genes control development along the anterior–posterior axis How is mesoderm in the anterior part of a mouse embryo programmed to produce forelegs rather than hind legs? In Section 19.5, we saw how homeotic genes control body segmentation in Drosophila. We also learned that all homeotic genes contain a DNA sequence called the homeobox. Some of the genes directing gastrulation in the frog are homeobox genes—for example, goosecoid and siamois. In vertebrates, the homeotic genes that control differentiation along the anterior–posterior body axis are called Hox genes. 44.15 Developing Body Segmentation (A) Repeating blocks of tissue called somites form on either side of the neural tube. Muscle, cartilage, bone, and the lower layer of the skin form from the somites. (B) In this SEM of somite formation in a chick embryo, the overlying ectoderm has been removed and the neural tube and somites are seen from above. In mammals, four Hox gene complexes reside on different chromosomes in clusters of about 10 genes each. Remarkably, the temporal and spatial expression of these genes follows the same pattern as their linear order on their chromosome. That is, the Hox genes closest to the 3′ end of each gene complex are expressed first and in the anterior of the embryo. The Hox genes at the 5′ end of the gene complex are expressed later and in a more posterior part of the embryo. As a result, different segments of the embryo receive different combinations of Hox gene products, which serve as transcription factors (Figure 44.16; see also Figure 20.2). Whereas Hox genes give cells information about their position on the anterior–posterior body axis, other genes provide information about their dorsal–ventral position. Tissues in each segment of the body differentiate according to their dorsal–ventral location. The notochord provides many of these signals. One example of a dorsal–ventral difference is seen in the spinal cord; sensory nerve connections develop in the dorsal region, and motor nerve connections in the ventral region. The protein Sonic hedgehog (named for a video-game character), which is expressed in the mammalian notochord, induces cells in the overlying neural tube (i.e., the ventralmost cells of the neural tube) to become motor neurons. After the development of body segmentation, the formation of organs and organ systems progresses rapidly. The development of an organ involves extensive inductive interactions of the kind we saw in the example of the vertebrate eye (see Figure 19.10). These inductive interactions are a current focus of study for developmental biologists. This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. The genes closest to the 3′ end are expressed in the anteriormost positions… 44.4 …and those closest to the 5′ end are expressed more posteriorly. b1 b2 b3 b4 b5 b6 b7 b8 b9 3′ 5′ Hoxb Expression gradients from anterior to posterior of embryo For example, Hoxb1 is expressed in the hindbrain… …and Hoxb9 in the spinal cord. Hindbrain Spinal co rd | HOW IS THE GROWING EMBRYO SUSTAINED? 937 Extraembryonic membranes form with contributions from all germ layers The chicken provides a good example of how extraembryonic membranes form from the germ layers created during gastrulation. In the chick, four membranes form—the yolk sac, the allantoic membrane, the amnion, and the chorion. The yolk sac is the first to form, and it does so by extension of the hypoblast layer along with some adjacent mesoderm. The yolk sac grows to enclose the entire body of yolk in the egg (Figure 44.17). It constricts at the top to create a tube that is continuous with the gut of the embryo. However, yolk does not pass through this tube. Yolk is digested by the cells of the yolk sac, and the nu- Midbrain Tho racic 5-Day chick embryo Lu m Forebrain ba r Cervical Embryo (head end) Mouse embryo 44.16 Hox Genes Control Body Segmentation Hox genes are expressed along the anterior–posterior axis of the embryo in the same order as their arrangement between the 3′ and 5′ ends of the gene complex. As a result of gene duplication during evolution, vertebrates have four copies of the Hox gene complex shown. Gut Amnion Amnionic cavity Chorion Yolk 44.3 RECAP Gastrulation sets up tissue interactions that initiate organogenesis. Neurulation is initiated by organizer mesoderm that forms the notochord. • Describe the formation of the neural tube in vertebrates. See p. 935 and Figure 44.14 • How do somites relate to segmentation of the body axis? See pp. 935–936 and Figure 44.15 • Using information from this chapter and from Chapters 19 and 20, explain what Hox genes are and how they instruct patterns of differentiation along the body axis. See Figures 19.19, 20.1, and 44.16 The first extraembryonic membrane is the yolk sac, which is forming in the 5-day embryo. The mesoderm and ectoderm extend beyond the embryo to form the chorion and the amnion. 9-Day chick embryo Embryo Gut Amnion Amnionic cavity Allantois Chorion You may be aware that in mammals the circulatory systems of the fetus and mother are separate and that nourishment reaches the fetus through the placenta and the umbilical cord. In the next section we will examine the developmental events that result in the creation of the placenta. 44.4 How is the Growing Embryo Sustained? There is more to a developing reptile, bird, or mammal than the embryo itself. As mentioned earlier, the embryos of these vertebrates are surrounded by several extraembryonic membranes, which originate from the embryo but are not part of it. Extraembryonic membranes function in nutrition, gas exchange, and waste removal. In mammals, they interact with tissues of the mother to form the placenta. Yolk Allantoic membrane Yolk sac The mesodermal and ectodermal layers fuse below the yolk so that the chorion lines the shell. Mesodermal and endodermal tissues form the allantois, a sac for metabolic wastes. 44.17 The Extraembryonic Membranes In birds, reptiles, and mammals, the embryo constructs four extraembryonic membranes. The yolk sac encloses the yolk, and the amnion and chorion enclose the embryo. Fluids secreted by the amnion fill the amniotic cavity, providing an aqueous environment for the embryo. The chorion, along with the allantoic membrane, mediates gas exchange between the embryo and its environment. The allantois stores the embryo’s waste products. yo u r B i oPort al.com GO TO Web Activity 44.1 • Extraembryonic Membranes This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 938 CHAPTER 44 | ANIMAL DEVELOPMENT 44.18 The Mammalian Placenta In humans and most other mammals, nutrients and wastes are exchanged between maternal and fetal blood in the placenta, which forms from the chorion and tissues of the uterine wall. The embryo is attached to the placenta by the umbilical cord. Embryonic blood vessels invade the placental tissue to form fingerlike chorionic villi. Maternal blood flows into the spaces surrounding the villi, and placental blood flows through the villi so nutrients and respiratory gases can be exchanged between the maternal and fetal blood. 2 months Fetus Amnion Chorion (fetal portion of placenta) Maternal portion of placenta Placenta Uterus Umbilical cord Amnion Umbilical arteries (from fetus) From fetus To fetus From fetus trients are transported to the embryo through blood vessels that form from mesoderm and line the outer surface of the yolk sac. The allantoic membrane is also an outgrowth of the extraembryonic endoderm plus adjacent mesoderm. It forms the allantois, a sac for storage of metabolic wastes. Ectoderm and mesoderm combine and extend beyond the limits of the embryo to form the other extraembryonic membranes. Two layers of cells extend all along the inside of the eggshell, both over the embryo and below the yolk sac. Where they meet, they fuse, forming two membranes, the inner amnion and the outer chorion. The amnion surrounds the embryo, forming the amniotic cavity. The amnion secretes fluid into the cavity, providing a protective environment for the embryo. The outer membrane, the chorion, forms a continuous membrane just under the eggshell (see Figure 44.17). It limits water loss from the egg and also works with the enlarged allantoic membrane to exchange respiratory gases between the embryo and the outside world. Extraembryonic membranes in mammals form the placenta In placental mammals, the first extraembryonic membrane to form is the trophoblast, which is already apparent by the fifth cell division (see Figure 44.4). When the blastocyst reaches the uterus and hatches from its encapsulating zona pellucida, the trophoblast cells interact directly with the endometrium. Adhesion molecules expressed on the surfaces of these cells attach them to the uterine wall. By secreting proteolytic enzymes, the trophoblast burrows into the endometrium, beginning the process of implantation (see Figure 44.5). Eventually, the entire trophoblast is within the wall of the uterus. The trophoblast cells then send out numerous projections, or villi, to increase the surface area of contact with maternal blood. Meanwhile, the hypoblast cells proliferate to form what in the bird would be the yolk sac. But there is no yolk in eggs of placental mammals, so the yolk sac contributes mesodermal tissues that interact with trophoblast tissues to form the chorion. The chorion, along with tissues of the uterine wall, produces the placenta, the organ that exchanges nutrients, respiratory Umbilical vein (to fetus) Chorionic villus Maternal vein (to mother) Maternal artery (from mother) gases, and metabolic wastes between the mother and the embryo (Figure 44.18). At the same time the yolk sac is forming from the hypoblast, the epiblast produces the amnion, which grows to enclose the entire embryo in a fluid-filled amniotic cavity. The rupturing of the amnion and chorion and the loss of the amniotic fluid (a process called “water breaking”) herald the onset of labor in humans. An allantois also develops in mammals, but its importance depends on how well nitrogenous wastes can be transferred across the placenta. In humans the allantois is minor; in pigs it is important. In humans and other mammals, allantoic tissues contribute to the formation of the umbilical cord, by which the embryo is attached to the chorionic placenta. It is through the blood vessels of the umbilical cord that nutrients and oxygen from the mother reach the developing fetus, and wastes, including carbon dioxide and urea, are removed (see Figure 44.18). 44.4 RECAP The extraembryonic membranes of reptiles, birds, and mammals sustain the growing embryo. In reptiles and birds, these membranes surround the embryo within the shelled egg. In mammals the extraembryonic membranes form the placenta, an organ that exchanges nutrients, respiratory gases, and metabolic wastes between the mother and the embryo. • Describe each of the four extraembryonic membranes and their functions in the developing chick egg. See pp. 937–938 and Figure 44.17 • Explain the role of the trophoblast in the early development of a mammalian embryo. See p. 938 This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 44.5 44.5 What Are the Stages of Human Development? In humans, gestation, or pregnancy, lasts about 266 days, or 9 months. In smaller mammals gestation is shorter—for example, 21 days in mice—and in larger mammals it is longer—for example, 330 days in horses and 600 days in elephants. The events of human gestation can be divided into three periods of roughly 3 months each, called trimesters. | WHAT ARE THE STAGES OF HUMAN DEVELOPMENT? 939 diation, drugs, chemicals, and pathogens that can cause birth defects. An embryo can be damaged before the mother even knows she is pregnant. A classic and tragic case is that of thalidomide, a drug widely prescribed in Europe in the late 1950s to treat nausea. Women who took this drug in the fourth and fifth weeks of pregnancy, when the embryo’s limbs are beginning to form, gave birth to children with missing or severely malformed arms and legs. Organ development begins in the first trimester Organ systems grow and mature during the second and third trimesters Implantation of the human blastocyst begins about 6 days after fertilization. After implantation, gastrulation occurs, tissues differentiate, the placenta forms, and organs begin to develop. The heart begins to beat during week 4, and limbs are formed by week 8 (Figure 44.19 A,B). By the end of the first trimester, most organs have started to form. The embryo is about 8 centimeters long and weighs about 40 grams (less than 2 ounces); it would fit neatly in a teaspoon. At about this point in time, the human embryo is medically and legally referred to as a fetus. (This distinction is not made for other mammals; developing mice, for example, remain embryos until they are born.) The first trimester is a time of rapid cell division and tissue differentiation. Signal transduction cascades and the resulting branching sequences of developmental processes are in their early stages. Therefore, the first trimester is the period during which the embryo is most sensitive to damage from ra- During the second trimester the fetus grows rapidly to a weight of about 600 grams. The limbs of the fetus elongate, and the fingers, toes, and facial features become well formed (Figure 44.19C). Eyebrows and fingernails grow and the fetus’s nervous system develops rapidly. Fetal movements are first felt by the mother early in the second trimester, and they become progressively stronger and more coordinated. The fetus grows rapidly during the third trimester (Figure 44.19D). As the trimester approaches its end, internal organs mature. The digestive system begins to function, the liver stores glycogen, the kidneys produce urine, and the brain undergoes cycles of sleep and waking. A human infant is born as soon as the last of its critical organs—the lungs—mature. Although the first-trimester embryo is the most susceptible to adverse effects of drugs, chemicals, and diseases, the potential for serious effects from exposure to environmental factors (A) 4 weeks (C) 4 months Actual length ~0.4 cm (4 mm) Actual length ~10 cm (B) 8 weeks (D) 9 months Actual length ~3 cm Actual length ~40 cm 44.19 Stages of Human Development (A) At 4 weeks of gestation, most of the embryo’s organ systems have been formed and the heart is beating. (B) The body structures of this 8-week-old embryo are forming rapidly, and it is visibly a male. The umbilical cord attaches the embryo to the placenta (upper left). (C) At 4 months, the fetus has fully formed limbs with fingers and toes, and moves freely within the amniotic cavity. (D) This fetus is well along in its ninth month. Soon its lungs will be mature enough to trigger the onset of contractions and birth. This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. CHAPTER 44 940 | ANIMAL DEVELOPMENT exists throughout pregnancy and continues after birth. Severe protein malnutrition, alcohol, and cigarette smoke are examples of factors that can cause low birth weight, mental retardation, and other developmental complications. Developmental changes continue throughout life Development does not end with birth. Obviously, growth continues until adult size is reached, and even when growth stops, organs of the body continue to repair and renew themselves through cycles of cell replacement by the progeny of undifferentiated stem cells. In humans especially, enormous developmental changes occur in the brain in the years between birth and adolescence. Especially in the early years, there is a great deal of plasticity in the organization of the nervous system as the connections between neurons develop. For example, a child born with misaligned eyes (a condition known as strabismus) will use mostly one eye. The connections to the brain from that eye will become strong while connections from the other eye remain weak, and the child will develop with reduced visual acuity and depth perception. If eye alignment is corrected in the first 3 years of life, the connections between the eyes and the brain can improve and the child is likely to develop normal vision. After the age of 3, correcting the connections between the eyes and the brain is less likely to result in improvement, and visual impairments may persist. Thus plasticity in human visual system development declines during early childhood. However, recent data indicate that it is not lost entirely and may be reactivated even in adulthood. 44.5 RECAP Human gestation lasts 9 months and can be divided into 3 trimesters. At the end of the first trimester, the fetus is very small but most of its organs have begun to form. In the second trimester, limbs elongate and the fetus moves. By the end of the third trimester, most organs have begun to function. • Why is a first-trimester embryo particular sensitive to environmental risks? See p. 939 CHAPTER SUMMARY 44.1 • • How Does Fertilization Activate Development? The sperm and the egg contribute differentially to the zygote. The sperm contributes a haploid nucleus and, in most species, a centriole. The egg contributes a haploid nucleus, nutrients, ribosomes, mitochondria, mRNAs, and proteins. In amphibians, the cytoplasmic contents of the egg are not distributed homogeneously, and they are rearranged after fertilization to set up the major axes of the future embryo. The nutrient molecules are generally found in the vegetal hemisphere, whereas the nucleus is found in the animal hemisphere. 44.2 • Cleavage is a period of rapid cell division. Except in mammals, little if any gene expression occurs during cleavage. Cleavage can be complete or incomplete, and the pattern of cell divisions depends on the orientation of the mitotic spindles. The result of cleavage is a ball or mass of cells called a blastula. Review • Early cell divisions in mammals are unique in being slow and allowing for gene expression early in the process. These cell divisions produce a blastocyst composed of an inner cell mass that becomes the embryo and an outer cell mass that becomes the trophoblast. At the time of implantation, the trophoblast secretes molecules that help the blastocyst implant in the uterine wall. Review Figures 44.4 and 44.5 A fate map can be created by labeling specific blastomeres and observing what tissues and organs are formed by their progeny. Review Figure 44.6 • Some species undergo mosaic development, in which the fate of each cell is determined during early divisions. Other species, including vertebrates, undergo regulative development, in which remaining cells can compensate for cells lost in early cleavages. The initial step of sea urchin and amphibian gastrulation is inward movement of certain blastomeres. The site of inward movement becomes the blastopore. Cells that move into the blastula become the endoderm and mesoderm; cells remaining on the outside become the ectoderm. Cytoplasmic factors in the vegetal pole cells are essential to initiate development. Review Figures 44.7 and 44.8 • The dorsal lip of the amphibian blastopore is a critical site for cell determination. It has been called the primary embryonic organizer because it induces determination in cells that pass over it during gastrulation. Review Figures 44.8, 44.9, and • The protein β-catenin activates a signaling cascade that induces the primary embryonic organizer and sets up the anterior–posterior body axis. Review Figures 44.2, and 44.11 Gastrulation in reptiles and birds differs from that in sea urchins and frogs because the large amount of yolk in reptile and bird eggs causes the blastula to form a flattened disc of cells. Figure 44.3 • Gastrulation involves massive cell movements that produce three germ layers and place cells from various regions of the blastula into new associations with one another. Review Figure 44.7, ANIMATED TUTORIAL 44.1 • Review Figures 44.1 and 44.2 • How Does Gastrulation Generate Multiple Tissue Layers? 44.10, ANIMATED TUTORIAL 44.2 • Review Figure 44.13 • Although their eggs have no yolk, placental mammals have a pattern of gastrulation similar to that of reptiles and birds. 44.3 • How Do Organs and Organ Systems Develop? Gastrulation is followed by organogenesis, the process whereby tissues interact to form organs and organ systems. This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. CHAPTER SUMMARY • • • In the formation of the vertebrate nervous system, one group of cells that migrates over the blastopore lip is determined to become the notochord. The notochord organizes the overlying ectoderm to thicken, form parallel ridges, and fold in on itself to form a neural tube below the epidermal ectoderm. The nervous system develops from this neural tube. Review Figure 44.14 The notochord and neural crest cells participate in the segmental organization of mesoderm into structures called somites along the body axis. Rudimentary organs and organ systems form during these stages. Review Figure 44.15 In vertebrates, Hox genes determine the pattern of anterior–posterior differentiation along the body axis in mammals. Other genes, such as sonic hedgehog, contribute to dorsal–ventral differentiation. Review Figure 44.16 44.4 • • How is the Growing Embryo Sustained? The embryos of reptiles, birds, and mammals are protected and nurtured by four extraembryonic membranes. In birds and rep- tiles, the yolk sac surrounds the yolk and provides nutrients to the embryo, the chorion lines the eggshell and participates in gas exchange, the amnion surrounds the embryo and encloses it in an aqueous environment, and the allantois stores metabolic wastes. Review Figure 44.17, WEB ACTIVITY 44.1 In mammals, the chorion and the trophoblast cells interact with the maternal uterus to form a placenta, which provides the embryo with nutrients and gas exchange. The amnion encloses the embryo in an aqueous environment. Review Figure 44.18 44.5 • • 941 What Are the Stages of Human Development? Human pregnancy, or gestation, can be divided into three trimesters. The embryo forms in the first trimester; during this time, it is most vulnerable to environmental factors that can lead to birth defects. During the second and third trimesters the fetus grows, the limbs elongate, and the organ systems mature. Development continues throughout childhood and throughout life. SELF-QUIZ 1. Fertilization involves all of the following except a. equal contributions of cell organelles from sperm and egg. b. joining of sperm and egg haploid nuclei. c. induction of rearrangements of the egg cytoplasm. d. sperm binding to specific sites on the egg surface. e. metabolic activation of the egg. 2. Which of the following does not occur during cleavage in frogs? a. A high rate of mitosis b. Reduction in the size of cells c. Expression of genes critical for blastula formation d. Orientation of cleavage planes at right angles e. Unequal division of cytoplasmic determinants 3. How does cleavage in mammals differ from cleavage in frogs? a. Slower rate of cell division b. Formation of tight junctions c. Expression of the embryo’s genome d. Early separation of cells that will not contribute to the embryo e. All of the above 4. Which statement about gastrulation is true? a. In frogs, gastrulation begins in the vegetal hemisphere. b. In sea urchins, gastrulation produces the notochord. c. In birds, cells from the surface of the blastodisc move down through the primitive groove to form the hypoblast. d. In mammals, gastrulation occurs in the hypoblast. e. In sea urchins, gastrulation produces only two germ layers. 5. Which of the following was a conclusion from the experiments of Spemann and Mangold? a. Cytoplasmic determinants of development are homogeneously distributed in the amphibian zygote. b. In the late blastula, certain regions of cells are determined to form skin or nervous tissue. c. The dorsal lip of the blastopore can be isolated and will form a complete embryo. d. The dorsal lip of the blastopore can initiate gastrulation. e. The dorsal lip of the blastopore gives rise to the neural tube. 6. Which of the following is true of human development? a. Most organs begin to form during the second trimester. b. Gastrulation takes place in the oviducts. c. Genetic diseases can be detected by sampling cells from the chorion. d. Implantation occurs through interactions of the zona pellucida with the uterine lining. e. Exposure to drugs and chemicals is most likely to cause birth defects when it occurs in the third trimester. 7. Which of the following characterizes neurulation? a. The notochord forms a neural tube. b. The neural tube is formed from ectoderm. c. A neural tube forms around the notochord. d. The neural tube forms somites. e. In birds, the neural tube forms from the primitive groove. 8. Which statement about trophoblast cells is true? a. They are capable of producing monozygotic twins. b. They are derived from the hypoblast of the blastocyst. c. They are endodermal cells. d. They secrete proteolytic enzymes. e. They prevent the zona pellucida from attaching to the oviduct. 9. Which of the following is part of the embryonic contribution to placenta formation? a. Amnion b. Chorion c. Ectoderm d. Allantois e. Zona pellucida 10. When is the developing human most susceptible to the occurrence of birth defects from radiation or chemical insults? a. At the time of birth b. During the third trimester c. During the first trimester d. When it is a zygote e. During the final stages of organ formation This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc. 942 CHAPTER 44 | ANIMAL DEVELOPMENT FOR DISCUSSION 1. If you found a protein that was localized to a small group of cells in the frog blastula, how would you determine whether that protein played a role in development? Address the issues of sufficiency and necessity. 2. During gastrulation in birds, the sonic hedgehog gene is expressed only on the left side of Hensen’s node. What might be the cause of this expression pattern, and what is its significance? 3. Much of the early work of describing animal development was done on sea urchins, amphibians, and chickens. Most of the recent work on the molecular mechanisms of animal development has been done on nematodes, fruit flies, zebrafish, and mice. Why do you think there has been a shift in the animal models used by developmental biologists? 4. If all the mitochondria and mitochondrial DNA in the embryo come from the egg, what implications does this have for using mitochondrial DNA for molecular evolutionary studies? 5. There is currently much controversy over therapeutic cloning as a way of obtaining embryonic stem cells to treat diseases. Given that human development is regulative—in other words, twinning can occur if an early blastocyst is divided into two cell masses—can you think of a way to guarantee a source of isogenic (i.e., identically matching a person’s own body) stem cells for an individual without resorting to therapeutic cloning? Assume isolated cells can be preserved indefinitely in a frozen state. A D D I T I O N A L I N V E S T I G AT I O N It is hypothesized that the differential development of the different body segments is due to the differential expression of Hox genes along the anterior–posterior body axis. For example, in mammals, ribs develop in anterior body trunk segments but not in posterior segments. Using the mouse as a model, how would you test this hypothesis? This material cannot be copied, reproduced, manufactured, or disseminated in any form without express written permission from the publisher. © 2010 Sinauer Associates, Inc.