to view Pediatric T1DM Case Study
advertisement

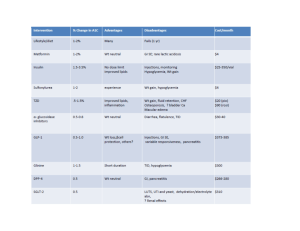
Aubrey Burklin Pediatric T1DM case study 1.what are the current thoughts regarding the etiology of type 1 DM? No one else in Rachel’s family has diabetes- is this unusual? Are there any other findings in her family medical history that would be important to this note? (Krause, Escott-Stump) The primary defect in T1DM is pancreatic beta cell destruction, usually leading to absolute insulin deficiency. There are two forms of T1DM immune-mediated and idiopathic. Immune-mediated DM results from an autoimmune destruction of the beta cells of the pancreas, the only cells in the body that make the hormone insulin. The onset of immune-mediated DM often follows viral infection. Idiopathic T1DM refers to forms of the disease that have no known cause, found mostly in people of African or Asian origin. It is not unusual or unheard of for someone to develop T1DM when there is no familial history. If someone in your direct family (siblings, parents) has diabetes it does increase your risk. Your risk is also increased if someone in your family has an autoimmune disease. It would be important to note her family has a history of an autoimmune disease, or a polyglandular condition because that increases her risk of having T1DM. Her mother has hyperthyroidism and her sister has celiac disease. This puts Rachel at an increased risk for having T1DM. 2.What are the standard Diagnostic criteria for T1DM? Which are found in Rachel’s medical record? (Krause, Escott-Stump) For diagnosis of diabetes, a fasting plasma glucose (FPG) test or an oral glucose tolerance test can be used to identify diabetes. But four diagnostic measures may be used to diagnose diabetes and each, in the absence of unequivocal hyperglycemia, must be confirmed on a subsequent day by repeat testing. ! FPG≥ 126mg/dL or ! 2-hr PG≥ 200mg/dL during an OGTT or ! HbA1C ≥ 6.5% or ! In patients with classic symptoms of hyperglycemia or hyperglycemic crisis, a random PG≥ 200mg/dL Rachel was admitted to the hospital after she fainted, when conscious reported recent wt. loss, polydipsia, polyuria, polyphagia. The day she was admitted her blood glucose level was 683mg/dL well above the normal limit of 110. Rachel’s HbA1C on the day she was admitted was 14.6% well above the normal limit of 5.2%. There were also ketones present in her urine. 3. Using the information from Rachel’s medical record, identify risk factors that would allow the physician to distinguish between T1DM and T2DM. (Krause, Escott-Stump, Mayo clinic) ! Clinical onset of the diabetes was abrupt ! Rachel’s is young 12y.o. and affected persons with T1DM are usually children and young adults although it can occur at any age. ! Has tested positive for IAA , the day she was admitted. IAA is usually the first marker to appear in children, is an antibody that is targeted to insulin. Insulin is the only antigen thought to be highly specific for beta cells ! She was positive for islet cell antibodies( +ICA) ! She was positive for GADA ! She has recently experienced weight loss ! Has experienced polydipsia, polyuria, polyphagia ! Fainted in the middle of soccer practice, most likely d/t hyperglycemia ! Has ketones present in her urine indicating her cells have been unable to utilize glucose and has had to rely on breakdown of fat as energy source for cell, ketones are a byproduct of this fat breakdown. ! She has a lower than average c peptide value which means that her proinsulin (inactive) is cut into insulin (active) + c peptide at an amount that is lower than a normal persons, d/t the majority of her beta cells being destroyed. 4. Describe the metabolic events that led to Rachel’s symptoms and subsequent admission to the ER (polyuria, polydipsia, polyphagia, fatigue, weight lost), integrating the pathophysiology of T1DM into your discussion. (Society for Neuroscience. "How Inflammatory Disease Causes Fatigue." http://www.sciencedaily.com/releases/2009/02/090217173034.html) Severe and extensive pancreatic beta cell damage is mediated by the immune system of Rachel’s body. As glucose floods the blood stream from the gut, it passes the pancreas which monitors glucose levels. The pancreas would normally release from the beta cells an adequate amount of insulin to Trigger GLUT 4 receptors uptake of glucose into cells (in peripheral tissue), but d/t the extent of the damage to the beta cells, very little insulin is produced from the pancreas, in response to a high carbohydrate meal. This means that the blood glucose level stays elevated, and cells are not delivered the adequate amount of glucose to function. Cells are literally starved for energy, this intracellular starvation causes polyphagia. Also according to the gluco-stat theory if insulin is low then glucose is not able to trigger the satiety center so the feeding center stays triggered also leading to polyphagia. Intracellular starvation is occurring, so the body mobilizes fat stores to utilize for energy. Ketones are a byproduct of this process but when ketones begin to overload the system (similar to glucose) they are excreted in the urine. Insulin is also used to signal anabolic processes, and halt catabolic processes. Since there is not a sufficient amount of insulin present to suppress glucagon, glucagon is released and causes the liver to produce more glucose, and dump that into the blood adding to the already elevated blood sugar level. When the blood sugar level surpasses the renal threshold, glucose resorption from renal tubule does not completely happen, thusly glucose begins to appear in the urine. So now you’ve got glucose in the urine causing the osmotic pressure to be raised, which will slow kidney resorption of water d/t the high solute concentration of glucose in the nephron, which means polyuria (increased urination) and fluid loss. This increased urination and fluid loss leads to dehydration, in your mind this triggers the hypothalmic thirst center and causes polydipsia excessive thirst. Weight loss may be due to dehydration and fluid loss as well as from loss of fat that the body is utilizing for energy. Fatigue may be due to frequently disrupted sleep to use the restroom, high blood glucose slows the flow of blood and thus delivery of nutrients and oxygen to cells, intracellular starvation leading to energy deficit, high blood sugar may cause inflammation of blood vessels triggering monocytes which infiltrate the brain this May cause fatigue. 5. Describe the metabolic events that result in the signs and symptoms associated with DKA. Was Rachel in this state when she was admitted? What precipitating factors may lead to DKA? (Krause 703) Signs and symptoms associated with DKA: hyperglycemia can lead to DKA, DKA is always the result of inadequate insulin for glucose use, DKA is characterized by elevated blood glucose, the presence of ketones in the urine and blood. Symptoms include polyuria, polydipsia, hyperventilation, dehydration, the fruity odor of breath and fatigue. Despite hyperglycemia, cells are in a state of intracellular starvation d/t the fact that there is not sufficient insulin (majority of beta cells are destroyed) to trigger GLUT 4 translocation to the cell membrane to allow for the uptake of glucose into the cell. The body in an attempt to fuel the starving cells, begins to mobilize TG’s. One of the byproducts of mobilizing a large amount of TG’s is ketone bodies (acetoacetic acid and beta hydroxybutyric acid). Large amounts of ketone bodies present in the serum and urine can be dangerous. Ketosis and Acidosis results from increased production and decreased utilization of ketone bodies (essentially an accumulation of acidic products in the blood). Recap, the blood glucose level is high, and there are a large amount of ketone bodies present in the blood, which result in a drop in pH. The kidneys then attempt to eliminate glucose and ketones. When the blood sugar level surpasses the renal threshold, glucose resorption from renal tubule does not completely happen, thusly glucose begins to appear in the urine. So now you’ve got glucose and ketones in the urine causing the osmotic pressure to be raised, which will slow kidney resorption of water d/t the high solute concentration of glucose and ketones in the nephron, which means polyuria (increased urination) and fluid loss. This increased urination and fluid loss leads to dehydration, in your mind this triggers the hypothalmic thirst center and causes polydipsia, excessive thirst. Weight loss may be due to dehydration and fluid loss as well as from loss of fat that the body is utilizing for energy. Fatigue may be due to frequently disrupted sleep to use the restroom, high blood glucose slows the flow of blood and thus delivery of nutrients and oxygen to cells, intracellular starvation leading to energy deficit, high blood sugar may cause inflammation of blood vessels triggering monocytes which infiltrate the brain this May cause fatigue. The lungs in an attempt to balance out the acidosis will increase ventilation rate and subsequent elimination of CO2 (hyperventilation) resulting in raising of the pH level. Acetoacetic acid (ketone body) may spontaneously decarboxylate to acetone, which like ketones may be eliminated through exhalation or the urine. It is the acetone that is responsible for the fruity smelling breath. The day Rachel was admitted she did have ketones present in her urine, she did complain of fatigue, polydipsia, polyuria. I don’t see a value for ABG to indicate acidity, but her urine was acidic. Nutrition and Diagnosis related care e7 states that “Diagnosis of DKA requires the patient’s plasma glucose concentration to be greater than 250mg/dL, pH level to be less than 7.30, and bicarbonate level to be less than or equal to 18mEq/L.” I think requesting for an additional test to determine DKA would be reasonable in attempt to confirm. I think it seems plausible that Rachel was in this state BUT I personally wouldn’t feel comfortable treating her for DKA if I didn’t have the lab tests to confirm the diagnosis. I suppose it would depend on the procedures of the facility that I worked in, ultimately. I would say that the number one precipitating factor is physiological stress, for example acute and severe infection or illness, possibly even sever emotional stress. When adequate insulin is no longer available glucose production is stimulated by the counter regulatory hormones, via lipolysis and gluconeogenesis to avoid intracellular starvation. Then glucose and ketones build up to such a high degree (subsequent hyperglycemia and metabolic acidosis) and achieve osmotic diuresis, which results in dehydration and electrolyte imbalance. Then fluid is lost, resulting in the blood becoming more concentrated which causes hyperglycemia. Also if you’re sick it can throw off your schedule and lead to people not taking their injections. 6.Rachel will be started on a combination of Apidra prior to meals and snacks with glargine given in the am and pm. Describe the onset, peak, and duration for each of these types of insulin. Her discharge dosages are as follows: 7 u glargine with Apidra prior to each meal or snack- 1:15 insulin: carbohydrate ratio. Rachel’s parents want to know why she cannot take oral medications for her diabetes like some of their friends do. What would you tell them? (Krause 696,692) Apidra is short acting, rapid onset insulin. Has an onset of action within 15 minutes, a peak in activity 60 to 90 minutes (or two hours), and a duration of 3 to 5 hours. Glargine is a long acting insulin. Has a slow rate of dissolution at the injection site, this results in a relatively constant peakless delivery over 24 hours. Must be given at a consistent time day to day if given before meals. Onset of action is 2-4 hours, it is peakless, it’s effective duration is 20-24 hours. T1DM may only be treated with insulin, which needs to be injected. It may not be given orally because it is a protein and our stomach acid would break it down before our intestines would have a chance to absorb it. I suspect their friends are T2DM which has many oral options for drug treatment before progressing to insulin dependency. 7. Rachel’s physician explains to Rachel and her parents that Rachel’s insulin dose may change due to something called a honeymoon phase. Please explain what this is and how it might affect her insulin requirements. The honeymoon phase is the phase where endogenous insulin secretion recovers after correction of: hyperglycemia, metabolic acidosis, and ketoacidosis. During this honeymoon phase exogenous insulin requirements decrease dramatically for up to 1 year or longer, and good metabolic control may be easily achieved. However, the need for increasing exogenous insulin replacement is inevitable and should always be anticipated. (Krause 678) 8. How does physical activity affect blood glucose levels? Rachel is a soccer player and usually plays daily. What recommendations will you make to Rachel to assist with managing her glucose during exercise and athletic events? (Krause 688, 689) Physical activity has a mechanism independent of insulin that causes GLUT 4 translocation to the cell membrane to allow for uptake of glucose into the cell. This leads to a drop in blood glucose levels. I would recommend that Rachel ask her doctors permission before returning to soccer and that her blood glucose be under control. I want her and her parents to talk with the doctor about the fact that she is returning to exercise and may need to adjust insulin level accordingly I would also like for her to be aware of the symptoms of hypoglycemia and hyperglycemia in case either of these situations happen during or after exercise. I want her to understand that heat intolerance will affect her easily as a diabetic and that maintaining proper hydration is important. Before exercise I would like for Rachel to check her blood glucose level, if it is below 100mg/dL then I would like for her to follow the 15:15 rule. Ingest at least 15g of carbohydrates and wait 15minutes and then test her blood again. There is a general recommendation to add 15g of carbohydrate for every 30-60minutes of activity (variable by intensity of activity). Since exercise causes a general decline in blood glucose level gradually, ingesting carbohydrates during prolonged exercise can improve performance by maintaining availability and oxidation of blood glucose. So ingest carbohydrates after 40-60minutes of exercise is important and may also assist in preventing hypoglycemia. Drinks that contain 6% or less of carbohydrates are a quick way to deliver these carbs and stay hydrated during exercise. Eating carbohydrates immediately after exercise optimizes repletion of muscle and liver glycogen stores, this is important for diabetics because they have an increased risk for late onset hypoglycemia post exercise. 9. Rachel’s blood glucose records indicate that her levels have been consistently high when she wakes in the morning before breakfast. Describe the dawn phenomenon. Is Rachel experiencing this? How might it be prevented? (Krause 703) Fasting hyperglycemia is common in people with diabetes. The amount of insulin required to normalize blood glucose levels at night during the predawn period (1am-3am) is less than at dawn (4-8am). The increased need for insulin at dawn causes a rise in fasting blood glucose levels this is the dawn phenomenon. The dawn phenomenon results in insulin levels decline between predawn and dawn or if overnight hepatic glucose output becomes excessive. To identify the dawn phenomenon, blood glucose levels need to be taken at bedtime and at 2-3am. With the dawn phenomenon, predawn blood glucose levels will be in the low range of normal but not in the hypoglycemic range. It looks like Rachel is experiencing this but to be sure I would like for levels to be checked before going to bed, at 2-3am, and when she wakes up (this would be easier if she was still in the hospital but to confirm the dawn phenomenon This should be done) Since Rachel is T1DM administering an insulin that does not peak at 1-3am should be considered, such as a long-acting insulin (obviously something she needs to discuss with her doctor before altering), but I believe that she is already on the longer duration of the long acting insulin’s (considering the information available in the charts of the Krause book). Also by looking at question 20 I am curious if she is even checking her blood glucose before going to bed. It is not indicated on her chart. That does not necessarily mean she is not checking it but it is something I would want to address with Rachel and her parents. 10. The MD ordered a consistent carbohydrate-controlled diet when Rachel begins to eat. Explain the rationale for monitoring carbohydrate in diabetes nutrition therapy. ( krause702,697,685) Blood glucose levels after eating are primarily determined by the rate of appearance of glucose from carbohydrate digestion and absorption into the bloodstream and the ability of insulin to clear glucose from the circulation. The total amount of carbohydrate eaten at a meal is the primary determinate of postprandial glucose levels. Although numerous factors influence glycemic response to foods, monitoring total grams of carbohydrates remains a key strategy in achieving glycemic control. Dayto-day consistency in the amount of carbohydrate eaten at meals and snacks is reported to improve glycemic control, especially in persons on fixed insulin regimens. Insulin doses are (or should be) adjusted to match carbohydrate intake. If Rachel is monitored for how many carbohydrates she has eaten and her glycemic response, it will allow for an accurate dosing of insulin. 11. Outline the basic principles for Rachel’s nutrition therapy to assist in control of her T1DM. (Krause 683) Maintenance of normal growth and development, maintaining a healthy weight is the main overarching goal. Integrate an insulin regimen into Rachel’s usual eating habits and physical activity. Stress the importance of SMBG when wake up & before bed, before & after exercise. Would get her parents in with her during the nutrition education to talk about successful meal plan approaches, insulin dosing, SMBG, and carbohydrate counting vs. meal plan. Most of all I want to make sure whatever intervention choice we go with fits the family and their lifestyle the best of all options, and I want to ensure we discuss any possible barriers, and answer any questions Rachel or her parents may have. Carb distribution across the day, appropriate portion of carbohydrates in the meal, insulin dose to match carbohydrates, 12. Assess Rachel’s ht./age, wt./age, ht./wt., and BMI. What is her desirable weight? Used the CDC calculator for children (http://apps.nccd.cdc.gov/dnpabmi/Result.aspx?&dob=1/1/2002&dom=2/12/2014&age=145&ht=6 0&wt=82&gender=2&method=0&inchtext=0&wttext=0) For ht./age she is between the 50-75% For wt./age she is just above the 25% For ht./wt. she is For BMI she is at 16 which is at the 17th percentile within healthy weight range Desirable weight, go to the 50% percentile, that would put her at about 93lbs. 13. Identify and abnormal lab values measured upon her admission. Explain how they may be related to her newly diagnosed T1DM. Low Sodium levels may be explained by her polydipsia and drinking large quantities of water in response. A low sodium level in the blood may result from excess water or fluid in the body, diluting the normal amount of sodium so that the concentration appears low. High Glucose levels d/t her being T1DM and not having enough insulin to clear the blood of glucose. Low phosphate levels could be d/t her lungs compensating for blood pH and attempting to raise it via increased ventilation rate and elimination of CO2 (respiratory alkalosis). Decrease in blood CO2 which increases intracellular pH. The increase in pH stimulates phosphofructokinase which enhances glycolysis, causing phosphate to move into cells to supply the enhanced phosphorylation that results. Could be d/t her insulin administration. Could be d/t increased renal loss. High Osmolality d/t the high blood glucose level, because she didn’t realize she was an uncontrolled T1DM High HbA1C HbA1C is a long-term measure of glycosylated hemoglobin it is high because she has had uncontrolled T1DM, and subsequent high levels of blood glucose. Low C-peptide d/t large amount of beta cell destruction. So proinsulin (inactive) is chopped into c-peptide+ insulin (active) in lower than usual amounts. Low insulin secretion parallels low c-peptide levels. ICA, GADA, IAA is present indicating that there has been an autoimmune destruction of pancreatic beta cells, these three antibodies are identified as contributing to the destruction of beta cells. High specific gravity concentrated urine has a high specific gravity value. Probably d/t the concentration of glucose and ketones Low urine pH d/t the concentration of acidic ketone bodies protein in urine d/t damaged glomeruli, that have an impaired ability to filter the blood and prevent protein from leaking into blood High glucose in urine d/t high blood glucose levels that surpass the renal thresh hold Ketones in urine indicate that the level of ketones in the body is so high (d/t utilizing fat as energy source) that they are being excreted in the urine Prot chk indicates that there is protein in her urine 14. Determine Rachel’s protein and energy requirements. Be sure to explain what standards you used to make this estimation. In persons with type 1 or type 2 diabetes with normal renal function, the RD should advise that usual protein intake of approximately 15 to 20% of daily energy intake does not need to be changed. Although protein has an acute effect on insulin secretion, usual protein intake in long-term studies has minimal effects on glucose, lipids, and insulin concentrations, if there is nephropathy damage though a protein intake of one gram or less per kg body weight per day is recommended. I found a paper by the ADA that says to use DRI for healthy kids http://care.diabetesjournals.org/content/28/1/186.full#ref-86 So I used the DRI formula from Krause and then multiplied it by a low active PAL (PAL Krause p26, formula p 27) 82/2.2= 37 rounded down 60x2.54=152.4 age=12 EER= 135.3-30.8 x age +PA x (10 x Wt.kg + 934 x ht m) +25 EER= [135.5- 30.8 x 12 + 1.4 x (10x 37 + 934 x 1.524) + 25 EER= 135.5 - 369.6 + 1.4 x ( 370 + 1,423.416) +25 EER= 2301.1824 Protein needs are 15-20% of daily needs (Nutrition and diagnosis related care e7) 2300x.15 = 345cal 2300x.20=460cal 345/4=86.25 460/4=115 range 86-115g protein daily 15. Prioritize two nutrition problems and complete the PES statement for each. (used IDNT book) Food and nutrition related knowledge deficit related to lack of exposure to information as evidenced by new diagnosis of diabetes, hyperglycemia blood glucose level of 683 mg/dL , HbA1C of 14.6% Impaired nutrient utilization related to polydipsia, polyuria, polyphagia, weight loss and fatigue as evidenced by lab results of 295.3 mmol/kg/H2O osmolality level, 683mg/dL glucose level, HbA1C 14.6%, weight loss of 9% in past month. 16. Determine Rachel’s initial nutrition prescription using her diet record from her home as a guideline, as well as your assessment of her energy requirements. Basic Nutrition Prescription (Krause 700) Food group Break fast Snack Lunch Snack Soccer pract Snack Dinner Total daily servings CHO Pro Fat cal starch 2 1 2-3 1 1 1 2-3 12 15 180 3 36 1 12 80 fruit 1 1 0-1 1 4 15 60 milk 1 1 1 3 12 36 8 24 veg 2-3 2-3 5 5 25 2 10 meat 2-3 3-4 6 60 7 42 1 3 100 25 5 30 75(55) fats 1 0-1 1-2 0-1 0 0-1 1-2 6 5 30 CHO choices 3-4 1 4-5 1 1 1 4-5 Total grams 301 112 75 Cal/ gram X4 1,204 X4 448 X9 675 Percent calories 52% 19% 29% 45 Total calories 2327 Basic meal plan was developed that can be tailored with the patient and parents. Discuss feasibility of meal plan, timing of meals and snacks with insulin, appropriate sizes of meals and snacks. Distribution of meals with snacks must be assessed along with the types of medication prescribed. Developed a plan that includes 11 starches, 4 fruits, 3 milks, 5 veggies, 6 meats/subs., 6 fats that calorically comes close to the Kcal average of 2300. Basic carbohydrate counting emphasizes the following topics: basic facts about carbohydrates, primary food sources of carbohydrates, average portion sizes, the importance of consistency and accurate portions, amount of carbohydrates that should be eaten, and label reading. An important goal of nutrition counseling is to facilitate changes in existing food and nutrition related behaviors and the adoption of new ones, through motivational interviewing, assessment of readiness to change, adapt the nutrition intervention plan to the clients lifestyle as much as possible, and agree upon set goals. For Rachel a lot of this would involve her parents since they are the caregivers and are most likely packing her lunches and preparing her dinners, they will also probably be helping with glucose monitoring and insulin administrations. 17. What is an insulin:CHO ratio (ICR)? Rachel’s physician ordered her ICR to start at 1:15. If her usual breakfast is 2- poptarts and 8oz of skim milk, how much Apidra should she take to cover the carbohydrate in this meal? Insulin carbohydrate ratio is the number of grams of carbohydrate covered by one unit of insulin. For example, if your insulin-to-carbohydrate ratio is 1:15, then you need to deliver one unit of insulin to cover every fifteen grams of carbohydrates you eat. ICR can be established for an individual by physician and will guide decisions on the amount of mealtime insulin to inject. www.supertracker.usda.gov/foodapedia used to find carbohydrates grams: 76g (2 poptarts) + 12g (8oz skim milk) so, 88gCHO /15=5.86 ≈6u 88g CHO 18. Dr. Cho set Rachel’s fasting blood glucose goal at 90-180mg/dL. If her total daily insulin does is 33u and her fasting a.m. blood glucose is 240mg/dL, what would her correction dose be? (Krause p. 693) CD= 1700/33= 51.5 about 52mm/dL. 1 unit of insulin should lower blood glucose level by Using a dose of 2 units of insulin should be enough to lower blood glucose to around 140mg/dL which is within her target range. Of 90-180mg/dL 19. Write an ADIME note for your initial nutrition assessment. Assessment Rachel Roberts Anthropometric Measurements: Age:12 Y.O. Sex: female Height: 5ft Weight: 82lbs. BMI: 16=17th % healthy Usual weight: 90lbs Admitted: w/ acute-onset hyperglycemia after fainting in soccer practice. Caucasian, catholic, student, English only Nutrition Focus Physical Findings: General appearance: Slim, healthy-appearing Skin: pale, diaphoretic Urine: Cloudy and Amber Vitals: Temp:98.6 Pulse:101 BP:122/77 Resp. rate:22 Pt. chief complaint: polyphagia, polydipsia, polyuria, wt. loss indicated by looser clothing MHX: None-recently had strep throat Meds: none at home SHX: nonsmoker, no alcohol use FHX: Father- HTN, Mother-Hypothyroidism, sister-celiac disease. Single 7th grade, Parents divorced, siblings sister 8, brother 4. Neurologic: alert but slightly confused Biochemical Data: Increased: Glucose, osmolality, HbA1C, +ICA, +GADA, +IAA, Specific gravity in urine, +protein in urine, +glucose in urine, +ketones in urine, +prot chk. In urine Decreased: sodium, phosphate, c-peptide, decreased pH in urine Food and Nutrition Related History: Usual Intake: Mom and Dad state that Rachel is kind of a picky eater. She eats only chicken and fish- eats salad, broccoli, carrots, tomatoes, and asparagus as her only vegetables. Breakfast-cereal and milk or pop-tart with milk; packs lunch for school- peanut butter and jelly or turkey and cheese sandwich, chips, carrots, and usually drinks water. Has cereal or granola bar before soccer practice- drinks water throughout practice. Dinner is usually prepared by Mom when she is at her house-always some salad, meat, and pasta, potato, or rice. Dad states that when the kids a re with him he doesn’t cook very often and they usually order pizza or Chinese food. Snacks include cereal, ice cream, yogurt, some fruits (apple, bananas), popcorn, chips, or cookies. Estimated needs: 2,300 Kcal Nutrition Diagnosis Food and nutrition related knowledge deficit related to lack of exposure to information as evidenced by new diagnosis of diabetes, hyperglycemia blood glucose level of 683 mg/dL , HbA1C of 14.6% Nutrition Intervention Basic Nutrition Prescription (Krause 700) Food group Break fast Snack Lunch Snack Soccer pract Snack Dinner Total daily servings CHO Pro Fat cal starch 2 1 2-3 1 1 1 2-3 12 15 180 3 36 1 12 80 fruit 1 0-1 1 4 15 60 1 60 milk 1 1 1 3 12 36 8 24 veg 2-3 2-3 5 5 25 2 10 meat 2-3 3-4 6 7 42 1 3 100 25 5 30 75(55) 5 30 45 fats 1 0-1 1-2 0-1 0 0-1 1-2 6 CHO choices 3-4 1 4-5 1 1 1 4-5 Total grams 301 112 75 Cal/ gram X4 1,204 X4 448 X9 675 Percent calories 52% 19% 29% Total calories 2327 Basic meal plan was developed that can be tailored with the patient and parents. Discuss feasibility of meal plan with , timing of meals and snacks with insulin, appropriate sizes of meals and snacks with Rachel and her parents. Distribution of meals with snacks must be assessed along with the types of medication prescribed. Developed a plan that includes 12 starches, 4 fruits, 3 milks, 5 veggies, 6 meats/subs., 6 fats that calorically comes close to the Kcal average of 2300. Basic carbohydrate counting emphasizes the following topics: basic facts about carbohydrates, primary food sources of carbohydrates, average portion sizes, the importance of consistency and accurate portions, amount of carbohydrates that should be eaten, and label reading. An important goal of nutrition counseling is to facilitate changes in existing food and nutrition related behaviors and the adoption of new ones, through motivational interviewing, assessment of readiness to change, adapt the nutrition intervention plan to the clients lifestyle as much as possible, and agree upon set goals. For Rachel a lot of this would involve her parents since they are the caregivers and are most likely packing her lunches and preparing her dinners, they will also probably be helping with glucose monitoring and insulin administrations. Nutrition Monitoring and Evaluation (Krause 702) Food intake, medication, metabolic control, anthropometric measurements, and physical activity should be monitored and evaluated. Medical and clinical outcomes should be monitored after the second and third visit to determine whether the patient is making progress toward established goals. If no progress is evident, the individual and RD need to reassess and perhaps revise nutrition interventions. Blood glucose monitoring results can be used to determine whether adjustments in foods and meals will be sufficient to achieve blood glucose goals or if medication additions or adjustment need to be combined with MNT. Nutrition care must be coordinated with an interdisciplinary team. Documentation in the patients medical record serves as a communication tool for members of the health care team, it also serves as a legal document of what was done and not done. Food records can be compared with the meal plan which will help to determine whether the initial meal plan needs changing and can be integrated with the blood glucose monitoring records to determine changes that can lead to improved glycemic control. 20. When Rachel comes to the clinic she brings her food and blood glucose record with her. A. Determine the amount of carbohydrates she is consuming at each meal. To get the carbohydrate grams I again utilized: www.supertracker.usda.gov/foodapedia 7:30 a.m. 2pop tarts (76 g) + 1 banana (27g) + 16 oz skim milk 16oz. (24g) +ovaltine 2tbsp. (9g) =136g CHO noon 2 slices pepperoni pizza (57g)+ 2 chocolate chip cookies-I used chips a hoy (13g) = 70g CHO 2p.m. granola bar (19g)= 19g CHO 4:30 p.m. Apple (19g) + 6saltines (13g) + 2tbsp. peanut butter (3g) = 35g CHO Soccer practice 16 oz Gatorade (31g)= 31g CHO 6:30 1cup rice (35g)+ 2oz chicken (o)+ 1/2c broccoli (17g)+ egg roll (19g)+ 2c skim milk (24g) =95g CHO 8:30p.m. 2c ice cream (63g)+ 2 tbsp peanuts (4g) = 67g CHO B. Determine whether she is taking adequate amounts of Apidra for each meal according to her record. 7:30a.m. 136g/15= 9.06 ≈9u Rachel gave herself 5u noon 70g/15=4.6≈ 5u rounded up to 5u because I am not sure if insulin is allowed to does at ½ units Rachel gave herself 6u 6:30p.m. 95g/15=6.3 ≈6u I rounded down again because I am not sure if insulin is allowed to be dosed in partial increments, also her BG was 80 which is the lowest we would ever want for it to drop because we don’t want her to slide into hypoglycemia. So with that being said I actually think that her dosing at 5u was appropriate considering that her BG was at the low end. 8:30p.m. 67g/15=4.46 ≈5u again I rounded d/t the fact that I am not sure that partial doses/ increments of insulin are given or not. (Rachel took 4u ) She did not note whether she took her blood glucose level before bed, make sure to talk with her about the importance of doing so. C. Calculate a correction dose for her to use. Using information from Jennifer Morgan’s power point : Normal weight persons with T1DM • Dosage: 0.5-1 unit/kg body wt. • Approx. 30-50% of the total daily insulin dose is used to provide for basal or background insulin needs • The remainder (bolus insulin) is divided among meals o Insulin-to-carbohydrate ratio o Proportionally to CHO content o 1-1.5 units/ 10-15g CHO consumed o Higher amount usually needed to cover breakfast carbohydrates Rachel is 82lbs ≈ 37kg 1unit x 37kg = 37u 37u x .4 = ≈15units basal "37-15= 21 units bolus so assuming 37 total daily insulin dose 1700/37=45.9 (using the correction factor in Krause) so one unit of insulin should lower the blood glucose by ≈ 45mg/dL