Lab Report Format: Science Experiment Template
advertisement

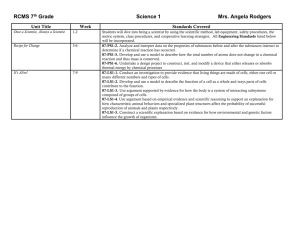
Lab Report Format Title Purpose Theory (Background) • Common Core 3.5.9-10A: “Cite specific text evidence to support an analysis of science texts” • Common Core 3.6.9-10C: “Provide clear and coherent writing appropriate to the task” Use your book or digital resources to gather the relevant information that is necessary to successfully complete the objective or purpose of the lab. Integrate this information into text that maintains the flow of ideas. At times, this section includes sample calculations. Be sure to cite your resources. Diagram/Photograph of the equipment set up used in the lab Data • Common Core 3.5.9-10G: “Translate quantitative information in words into a visual form: chart, table, graph)” This section typically includes a table/chart. It might also include BYOT photographs. Calculations Calculations should be labeled and then performed. Make sure your answer has the correct number of significant figures and units. Conclusion • Common Core 3.5.9-10I: Compare and contrast findings in a text to findings in other sources (i.e. lab results) The conclusion should start with a statement regarding the purpose. If you were to identify an unknown, state the identity here. Following that should be an indepth analysis of the errors in the lab. The sign on percent error is helpful here. If you have positive percent error, you have more than you should. If you have negative percent error, you have less than you should. Look at the data and the procedure to think of errors. T. O’Toole Group Practice Directions: Examine the following title and purpose statement from a lab. In your group discuss what types of information should be included in a theory section of a lab report. The terms are intentionally foreign to you so that you are forced to consider what a NOVICE scientist would need to know in order to understand the report. After discussion, use your textbook and/or digital research; integrate the appropriate information selectively into text so as to maintain the flow of ideas. Title: Empirical Formula of Copper (II) Chloride Purpose: Conduct a lab demonstrating proper measurement, analyze laboratory data, and mathematically determine the composition of a compound T. O’Toole Group Practice Directions: Examine the following title and purpose statement from a lab. In your group discuss what types of information should be included in a theory section of a lab report. The terms are intentionally foreign to you so that you are forced to consider what a NOVICE scientist would need to know in order to understand the report. After discussion, use your textbook and/or digital research; integrate the appropriate information selectively into text so as to maintain the flow of ideas. Title: Colligative Properties Purpose: Observe the effect of a non-volatile solute on the boiling point of water T. O’Toole Group Practice Directions: Examine the following title and purpose statement from a lab. In your group discuss what types of information should be included in a theory section of a lab report. The terms are intentionally foreign to you so that you are forced to consider what a NOVICE scientist would need to know in order to understand the report. After discussion, use your textbook and/or digital research; integrate the appropriate information selectively into text so as to maintain the flow of ideas. Title: Aluminum Lab Purpose: Perform a single replacement reaction, and calculate quantities using molarity and stoichiometry T. O’Toole Group Practice Directions: Examine the following title and purpose statement from a lab. In your group discuss what types of information should be included in a theory section of a lab report. The terms are intentionally foreign to you so that you are forced to consider what a NOVICE scientist would need to know in order to understand the report. After discussion, use your textbook and/or digital research; integrate the appropriate information selectively into text so as to maintain the flow of ideas. Title: Vinegar Titration Purpose: Titrate a sample of vinegar, graphically determine the endpoint of the titration, and calculate the percent acetic acid in vinegar T. O’Toole