#1 What is the molar mass of sucrose? (C H O ) 12.0 x 12 = 144.0
advertisement
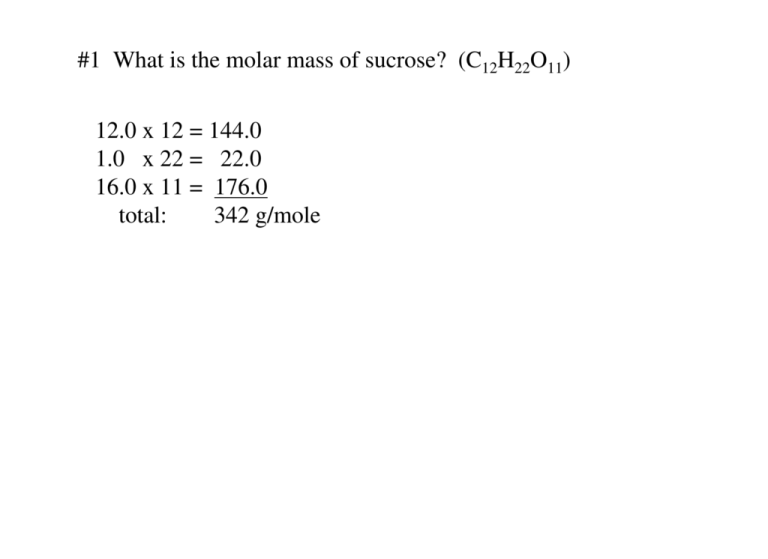
#1 What is the molar mass of sucrose? (C12H22O11) 12.0 x 12 = 144.0 1.0 x 22 = 22.0 16.0 x 11 = 176.0 total: 342 g/mole #2 What is the molar mass of each of the following compounds? a. Phosphorous pentachloride (PCl3) P; 31.0 Cl; 35.5 31 + (35.5 x 3) = 208.2 g/mole b. Uranium hexaflouride (UF6) U; 238.0.0 F; 19.0 238 + (19.0 x 6) = 352.0 g/mole #3 Calculate the molar mass of each of the following ionic Compounds: a. KMnO4 158.0 g/mole b. Ca3(PO4)2 310.2 g/mole #4 How many moles is 3.52 x 1024 molecules of water? 3.52 x 1024 molecules x 1 mole = 5.85 moles H2O 1 6.02 x 1023 molecules #5 How many atoms of zinc are in 0.60 moles of zinc? .60 moles x 1 6.02 x 1023 atoms = 1 mole 3.6 x 1023 atoms #6 What is the mass of 1.00 moles of oxygen (O2)? 1.00 moles x 1 32.0 grams 1 mole = 32.0 grams SECTION 2 MOLE-MASS and MOLE-VOLUME RELATIONSHIPS #1 What is the molar mass of each of the following compounds? a. C6H12O6 b. NaHCO3 c. C7H12 d. KNH4SO4 180.2 g/mole 84.0 g/mole 96.2 g/mole 153.2 g/mole #2 Calculate the mass in grams of each of the following: a. 8.0 moles lead oxide (PbO) 8.0 moles x 223.2 g = 1.8 x 10-3 g 1 b. .75 moles (H2S) 1 mole .75 moles x 34.1 g 1 1 mole c. .001 moles (SiH4) d. 1.50 x10-2 moles (O2) e. 2.30 moles (C2H6O2) = 25.6 g 3.2 x 10-2 g .48 g 1.43 x 102 g #3 How many grams are in 1.73 moles of dinitrogen pentoxide (N2O5) 1.73 moles x 1 (14.0 x 2) + (16.0 x 5) = 108 g/mole 108 grams = 1.87 x 102 grams 1 mole #4 How many grams are in .658 moles of calcium phosphate (Ca3(PO4)2 .658 moles x 1 310.3 g 1 mole = 204.2 grams #5 Calculate the number of moles in each of the following: 4.9 x 10-3 moles a. .50 g NaBr 9.10 x 10-2 moles b. 13.5 g Mg(NO3)2 c. 1.02 g MgCl2 1.08 x 10-2 moles d.. .00100 g CH3Cl 1.98 x 10-5 moles e. 1.50 x 10-3 g 1.97 x 10-5 moles C3H6(OH)2 #6 A chemist plans to use 435.0 grams of ammonium nitrate NH4NO3 in a reaction. How many moles of the compound is that? 435.0 g 1 x 1 mole 80 g = 5.43 moles #7 .0465 moles of C20H24N2O2 is how many grams? The molar mass is 12x20 + 24+ 28+ 32 = 324 g/mole .0465 moles x 324 g = 15.1 g 1 1 mole #8 What is the volume at STP of 2.66 moles of methane (CH4) gas? 2.66 moles x 22.4 liters 1 1 mole = 59.6 liters #9 How many moles is 135 L of ammonia (NH3) gas at STP? 135 liters x 1 mole = 6.03 moles NH3 1 22.4 liters 10.3 Percent Composition and Chemical formulas 1. A sample of a compound analyzed in a chemistry laboratory consists of 5.34 g of carbon, 0.42 g of hydrogen, and 47.08 g of chlorine. What is the percent composition of this compound? The total is: 52.84 g % comp. C is: 5.34 52.8 x 100 = 10.1% % comp. H is: .42 x 100 = .79% 52.8 % comp. Cl is: 47.08 52.8 x 100 = 89.1% #2 Find the percent composition of a compound containing tin and chlorine if 18.35 g of the compound contains 5.74 g tin. 5.74 g tin 18.35 x 100% = 31.3 % tin by difference you can get the % of chlorine: 100 % - 31.3 % = 68.7% chlorine #3 If 3.907 g of carbon combines completely with .874 g of hydrogen to form a compound, what is the percent composition of this compound? The total is: 4.781 % comp. C is: 3.907 4.781 x 100 = 81.7% % comp. H is: .874 x 100 = 18.3% 4.781 #4 From the formula for calcium acetate Ca(C2H3O2)2 calculate the mass of carbon that can be obtained from 65.3 g of the compound. The molar mass is: 40.1 + [(24+3+32) x 2] = 158.1 g mole The % comp. of Ca is: 40.1 x 100 = 25.3% 158.1 The % comp. of C is: (12x4) x 100 = 30.3% 158.1 The % comp. of H is: (1.0 x 6) x 100 = 3.79% 158.1 The % comp. of O is: (16.0 x 4) x 100 = 40.5% 158.1 Therefore: 65.3 g x .303 = 19.8 grams of carbon #5 How many grams of aluminum are in 25.0g of aluminum oxide Al2O3? The % composition of Al2O3 is: % comp. Al is: 54.0 (27.0 x 2) + (16x3) % comp. O is: 48.0 (27.0 x 2) + (16x3) x 100 = 52.9% x 100 = 47.1% Therefore the # of grams of Al in 25.0 g Al2O3 is: 25.0 x .529 = 13.2 g #6 How many grams of iron are in 21.6 g of iron (III) oxide (Fe2O3) First determine the % composition: % comp. Fe is: % comp. O is: 111.6 159.6 x 100 = 70.0% 48.0 159.6 x 100 = 30.0% Therefore 21.6 g x .70 = 15.11 grams #7 Determine the empirical formula for each of the following compounds from the percent composition: a. 7.8 % carbon and 92.2 % chlorine CCl 1. assume a 100 g sample 4 2. convert to moles 3. divide by the smallest number 4. multiply each number by a simple whole number (if necessary) to get whole number ratios. 7.8 % 7.8 g 92.2 % 92.2 g x 1 mole = .65/.65 = 1 12.0 g x 1 mole = 2.6/.65 = 4 35.5 g #7 Determine the empirical formula for each of the following compounds from the percent composition: b. 10.0 % carbon; .80 % H; 89.1 % chlorine 1. assume a 100 g sample 2. convert to moles 3. divide by the smallest number 4. multiply each number by a simple whole number (if necessary) to get whole number ratios. CHCl3 10.0 % 10.0 g .80 % .80 g 89.1 % 89.1 g x 1 mole = .83/.80 = 1 12.0 g x 1 mole = .80/.80 = 1 1.0 g x 1 mole = 2.51/.80 = 3.1 35.45 g







