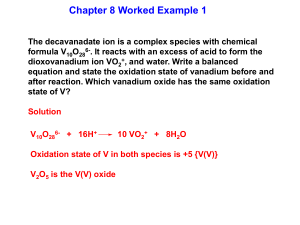
Transition Metal Coordination Chemistry
advertisement

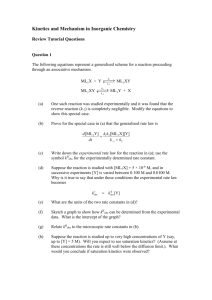
Transition Metal Coordination Chemistry What is a transition metal? Prof S.M.Draper 2.05 SNIAMS Building smdraper@tcd.ie Recommended books M.J. Winter, d-block Chemistry, Oxford Chemistry Primers, OUP, 2001 M.S. Silberberg, Chemistry, 3rd Ed, McGrawHill, 2003 (chapter 23) C.E. Housecroft, A.G. Sharpe, Inorganic Chemistry, 1st Ed, PrenticeHall, 2001 J.E. Huheey, E.A. Keiter, R.L. Keiter, Inorganic Chemistry, 4th Ed., HarperCollins, 1993 1 Electrons in atoms z Shapes of d-orbitals 4s y x 3d 4p Sc Ti z z z V y Cr y y Mn x x x Fe Co yz xz xy x2-y2 z2 Ni Cu Zn 2 Working out numbers of d-electrons from oxidation states: How many d-electrons has the metal? 1 2 3 4 5 6 7 8 9 10 11 12 1st: how many electrons are there in the shell? O - count along the periodic table e.g. Mn = 7 electrons ox = Cu = 11 electrons en = O -O H2N O- NH2 2nd: how many electrons are lost? - oxidation state complex O.S. of L O.S. of M no. d electrons e.g. Mn(VII) = 7 electrons lost Cu(II) = 2 electrons lost [Cr2O7]2- -2 +6 d0 [MnO4]- -2 +7 d0 0 +3 d1 -1, 0 +2 d8 -2 +3 d5 [Ag(NH3)2]+ 3rd: how many electrons left over? - subtract e.g. Mn(VII) = [Ti(H2O)6]3+ Cu(II) = [Co(en)3]3+ [PtCl2(NH3)2] [V(CN)6]4- Hence the only valance electrons available in a transition metal ion are d-electrons [Fe(ox)3]3- 3 Colour of transition metal complexes biological activity magnetic behaviour colour Ruby Corundum Al2O3 with impurities geometry What’s interesting about Sapphire Transition Metal Complexes?? Al2O3 with coordination number medical applications oxidation states octahedral metal centre Corundum and impurities coordination number 6 Emerald Beryl AlSiO3 containing Be with impurities 4 Haemoglobin Oxygen carrier in blood O2 Porphyrin-Fe transition metal complex Cisplatin [PtCl2(NH3)2] Fe(II) ion is octahedrally coordinated NN NN Fe NN Fe NN 2C HOHO 2C Coordination number 6 NR NR HO C 2 HO 2 C square planar Pt(II) coordination number 4 cis-isomer the first of a series of platinum coordination complex-based anti-cancer drugs (Platinol-AQ) 5 Alfred Werner - Nobel Prizewinner 1913 [Co(NH3)6]Cl3 [Co(NH3)5Cl]Cl2 3+ CoCl3 . 6NH3 yellow xs Ag+ 3 moles AgCl CoCl3 . 5NH3 purple xs Ag+ 2 moles AgCl CoCl3 . 4NH3 green xs Ag+ 1 mole AgCl xs Ag+ 0 moles AgCl CoCl3 . 3NH3 [Co(NH3)4Cl2]Cl + 2+ Werner's conclusions 1. The metal is in a particular (primary valancy) 2. The complex has a fixed (secondary valancy) 3. The ligands are bound to the metal via a bond which resembles a covalent bond 6 What is a coordination complex? Lewis Acid-Base Concept: parallel with main group chemistry • Adducts formed by the reaction of metal ions (Lewis acids) with several ligands (Lewis bases). H Ni2+ + H 2O H + 6 :NH 3 X+/- 2+ O . .: H 3 N: NH 3 n Ni NH 3 Ni 2+ n+/- Ni NH 3 2+ :NH 3 Central metal ion or atom surrounded by a set of ligands NH 3 The ligand donates two electrons to the d-orbitals around the metal forming a • Properties of products differ from those of separate reactants (i.e., colour, composition, magnetic properties, etc.). 1 3 7 F F zz F B z z F F B z z F z z H F B F H N H zz H F N H H NH3 _ H N > BF BF3 3 zz 6 L H N H L L 3+ zz 3 zz NH3 e.g. [Co(NH3)6]3+ z z + zz z z zz H H z z z z zz N z z H z z zz z z H + Co3+ H3N H3N z z z z z z NH3 z z NH3 zz L L NH3 L = coordinate or dative bond 1 C Group 14 O CO carbon monoxide e.g. NH3, H2O, OH-, CO32- CO, PPh3, C2H4, SRH, CN-, SCN- Small donor atoms Electronegative H H Group 15 N NH3 ammonia O H2O water H PPh3 phosphine S SR2 thioether Not very polarisable Less electronegative Easily polarisable R H Group 16 P Larger donor atoms H R e.g. Fe(III), Mn(II), Cr(III) e.g. Ag(I), Cu(I) Small metals (1st row) Larger metals (2nd + 3rd row) High oxidation state Low oxidation state 2 π - bonded ligands Group 14 C N z z CNcyanide - Phphenyl CH2 Cl K+ Cl H2C CH2 RC CR Pt Cl CH2 Group 15 N O z z Group 16 O Group 17 H X NOnitrous N OHhydroxide S halide C C H S NCS- z z N isocyanate z z [PtCl3(η2-C2H4)]- SCNthiocyanate hydride Monodentate donor atom per ligand Bidentate donor atoms per ligand Tridentate three donor atoms per ligand Multidentate many donor atoms per ligand 3 Anionic bidentate ligands Neutral bidentate ligands: 2 donor atoms O H 2N NH2 zz zz e.g. [PtCl2(en)] π-donor bidentate ligand O O O oxalate = ox2- - Fe C C O O O O 1,2-diaminoethane = ethylene diamine = en [Fe(CO)3(η4-C4H6)] acetate = ac- H 3C C O Ph2P zz PPh 2 zz N zz N zz N zz zz 2,2'-bipyridine bpy 1,10-phenanthroline phen R' O N O O Pd R' 1,2-diphenylphosphineethane dppe OH R N M O N HO R C N H N C H Pd(II)-oxime complex 4 Tetradentate ligands Tridentate ligands: three donor atoms Hexadentate ligand tetraanion of H 2N zz zz NH2 N NH2 NH zz N zz zz ethylenediaminetetraacetic acid N N NH2 zz EDTA NH2 O tris(2-aminoethyl)amine diethylenetriamine 2,2':6',2"-terpyridine dien tpy O - tren O N 1,2,4-triazacyclonane HN NH zz NH N HN N O N O O - O N N N zz macrocyclic ligand NH N O N HN N zz N H M porphyrin phthalocyanin 5 Inner coordination sphere = Outer coordination sphere = H2O SO42- H2O H2O Mn2+ OH2 OH2 H2O [Mn(OH2)6][SO4]: outer sphere complex H2O OSO3 H2O Mn2+ OH2 H2O H2O [Mn(OH2)5(SO4)5]: inner sphere complex 6 Ligand Exchange Reactions [Fe(OH2)6]2+ 2+ [Fe(OH2)5(CN)]+ + H2O + + hexaaquo iron(II) complex CN- + cyanide ion + K1 = const [H2O] = [Fe(OH2)5(CN)+] [H2O] [Fe(OH2)62+] [CN-] 1 The reaction continues…. The reaction continues…. [Fe(OH2)5(CN)]+ + K2 = CN- [Fe(OH2)4(CN)2]+ + H2O [Fe(OH2)4(CN)2] + CN- [Fe(OH2)3(CN)3]- + H2O β3 = [Fe(OH2)3(CN)3]- + CN- [Fe(OH2)2(CN)4]2- + H2O β4 = [Fe(OH2)2(CN)4]2- + CN- [Fe(OH2)(CN)5]3- + H2O β5 = [Fe(CN)6]4- + H2O β6 = [Fe(OH2)4(CN)2] [Fe(OH2)5(CN)+] [CN-] K1K2 = β2 [Fe(OH2)5(CN)+] = [Fe(OH2)62+] [CN-] x [Fe(OH2)3(CN)3-] [Fe(OH2)62+] [CN-]3 [Fe(OH2)2(CN)42-] [Fe(OH2)62+] [CN-]4 [Fe(OH2)(CN)53-] [Fe(OH2)62+] [CN-]5 [Fe(OH2)4(CN)2] [Fe(OH2)5(CN)+] [CN-] [Fe(OH2)(CN)5] + CN- Overall Stability Constant βn = [Fe(CN)64-] [Fe(OH2)62+] [CN-]6 [MLn] [M] [L]n Stability constants are often expressed in log form ie log βn 2 Values of βn The Chelate Effect 2+ 4- + 6 + 6 [M(NH3)6]2+ n+ NH3 H3N M NH3 NH3 H3N NH3 β6 = [Fe(CN)64-] [Fe(OH2)62+] [CN-]6 log β6 = 35 n+ H2N H2 N N H2 [M(en)3]2+ NH2 M NH2 H2N ~ 1035 [M(OH2)6]n+ + 6 NH3 [M(NH3)6]n+ + 6 H2O [M(OH2)6]n+ + 3 en [M(en)3]n+ 6 H2O + Kn < … < K3 < K2 < K1 3 ΔGo = ΔHo - TΔSo Entropy of chelate formation ΔGo = -RT ln K [Cu(OH2)6]2+ + β2107.7 ΔGo = ΔHo - TΔSo Enthalphy changes similar ΔHo = - 46 kJ + [M(OH2)6]n+ + mol-1 6 NH3 [M(NH3)6]n+ + 6 H2O 3 en [M(en)3]n+ + 6 H2O 2 H2O + 2 H2O log β2 = 7.7 ΔSo = - 8.4 J K-1mol-1 [Cu(OH2)4(en)]2+ en β2 1010.6 log β1 = 10.6 ΔHo = - 54 kJ mol-1 ΔSo is large and positive + Entropy changes differ [Cu(OH2)6]2+ + [M(OH2)6]n+ [Cu(OH2)4(NH3)2]2+ 2 NH3 ΔSo = + 23 J K-1mol-1 - TΔSo is large and negative 4 Thermodynamic vs Kinetic Stability The Macrocyclic Effect Complexes containing macrocyclic rings have enhanced stability compared to acyclic ligands 2+ H N N H 2+ H N H Cu Cu N H2 N N H2 log K1 = 23.9 acyclic chelating ligand H N N H log K1 = 28.0 macrocyclic ligand e.g [Cr(OH2)6]3+ d3 kinetically inert slow substitution of Ls cf. [Fe(OH2)6]3+ hs d5 kinetically labile rapid substitution of Ls (thermodynamic stability similar) ΔG always favours formation of macrocyclic complex 5 Tour of Coordination Numbers and their Geometries Most Common Geometries of Transition Metal Complexes The Kepert Model The shape of a complex is usually dictated by the number of coordinated atoms. The coordinated atoms are as far away from each other as possible. Tetrahedral 109o 28' C.N. 4 Square Planar 90o C.N. 4 Trigonal bipyramidal 120o + 90o C.N. 5 Square based pyramidal 90o C.N. 5 Octahedral 90o C.N. 6 Non- bonding electrons are ignored. Note: • regular geometries are often distorted • structural features of multinuclear complexes are described in terms used for individual metal centres • when energy differences between different structures are small, fluxional behaviour may be observed 6 Coordination number 2 Coordination number 5 Cu(I), Ag(I), Au(I), Hg(II) [CuCl2]- trigonal bipyramid [Au(CN)2]- square-based pyramid 180o 90o Coordination number 3 90o 120o [Cu(CN)2]- [HgI3]CN CN Cu Cu C N N C C 120o N N C Cu Cu CN CN The two structures have very similar energy n 7 trigonal bipyramid 3- Cl Cl Cu square-based pyramid Cl NC Cl CN CN [Co(CN)5]3- [CuCl5]3- + N Co Co NC Cl N 3- CN O N N Br [Co(Me6tren)Br]+ O O V O O [VO(acac)2] 8 Tetrahedral complexes Favoured by steric requirements optical isomerism 1 1 2 4 3 [CoCl4 4 3 ]2- [MnO4]90o 2 non-superimposable mirror images [NiCl4]2- 109o 1 Square Planar Geometry Square planar geometry e.g. [PtCl4]2[AuBr4][Co(CN)4]2- cisplatin Square planar complexes are formed by i.e. group 10 Ni2+, Pd2+, Pt2+ Au3+ cis-[PtCl2(NH3)2] trans-[PtCl2(NH3)2] cis-diamminedichloroplatinum(II) trans-diamminedichloroplatinum(II) Square planar complexes are formed by e.g. CN- 2 Coordination number 6 Octahedral geometry Geometrical Isomerism [ML4X2] e.g. [Mn(OH2)6]2+ [Cr(CO)6] Staggared ligands vs. Eclipsed ligands C+ trans-[Co(NH3)4Cl2]+ cis-[Co(NH3)4Cl2]+ green violet C+ do metals e.g. WMe6 3 Octahedral geometry [ML3X3] Geometrical Isomerism Octahedral geometry [M(Κ2L)2X2] Geometrical and Optical Isomerism e.g. [Co(en)2Cl2]+ trans geometrical isomer H N Cl H N Co N H Cl N H mer-[Co(NH3)3(NO2)3] cis geometrical isomer fac-[Co(NH3)3(NO2)3] two optical isomers Non-superimposable mirror images = Δ and Λ enantiomers 4 Octahedral geometry Optical Isomerism Tetragonal Distortion: Tetragonal elongation z-axis 2+ [M(Κ2L)3] 2 long axial & 4 shorter equatorial bonds N N Ru N N N N [Cu(OH2)6]2+, [Cr(OH2)6]2+ Tetragonal compression z-axis M [Ru(bpy)3]2+ 2 short axial & 4 longer equatorial bonds trans-[Co(NH3)4(CN)2]+ * Small distortion but symmetry lower than octahedral. 5 Isomerism Trigonal Distortion: • Two faces on opposite sides of octahedron move either towards or away from each other N N Rotate by 60° Elongation N Stereoisomerism Isomers have the same empirical Isomers have the same M-L bonds, formula but the atoms connectivities but the atoms are arranged differently differ in space Ionisation isomerism Geometrical isomerism M N N Complexes of tacn can show this type of distortion [M(tacn)2]2+ N Hydration isomerism cis/trans Coordination isomerism mer/fac Linkage isomerism N N Structural Isomerism N tacn = 1,3,7-triazacyclononane Polymerisation isomerism Optical isomerism Δ and Λ 6 Ionisation isomerism Coordination isomerism Exchange of a ligated anion with a counterion no ppt [Co(NH3)5Br]SO4 Ag+ [Co(NH3)5(SO4)]Br AgBr Ba2+ Ba2+ [Cu(NH3)4][PtCl4] [Pt(NH3)4][CuCl4] square planar [Co(NH3)6][Cr(CN)6] [Cr(NH3)6][Co(CN)6] octahedral BaSO4 no ppt Polymerisation isomerism Solvate isomerism Exchange of a neutral ligand for an anionic ligand e.g. [Co(OH2)6]Cl3 e.g. [Pt(NH3)4][PtCl4] [Pt(NH3)2Cl2] violet [Co(OH2)5Cl]Cl2 light green [Co(OH2)4Cl2]Cl Both polymers have the empirical formula [Pt(NH3)2Cl2]n dark green 7 Higher Coordination Numbers Linkage isomerism Coordination Number 7 hν [Co(NH3)5(NO2)]2+ Δ [Co(NH3)5(NO2)]2+ yellow red nitro-complex nitrito-complex [Pd(NCS)2(PPh3)2] isocyanate [Pd(SCN)2(PPh3)2] thiocyanate [WBr3(CO)4)](distorted) [TaF7]2[ZrF7]3- 8 Coordination Number 8 Na3[Mo(CN)8] (nBu4N)3[Mo(CN)8] Coordination Number 9 [ReH9]2- 9 Symmetrical ligand field z Shapes of d-orbitals orthogonal z y x M x and y z z z y y y x x x x2-y2 yz z2 xz xy Energy yz xz xy x2-y2 z2 x2-y2 yz z2 xz xy 1 Effect of ligand field on metal d-orbitals The difference in energy between the eg and the t2g energy levels is the . This is given the value 10 Dq. symmetrical field octahedral ligand field eg Δo or 10 Dq t2g 2 Electron configurations: d1 ion 3+ The nature of Δoct [Ti(OH2)6]3+ e.g. [Ti(OH2)6]3+ 490-580 nm violet solution in water one d-electron in a t2g orbital the complex has a Crystal Field Stabilisation Energy (CFSE) of - 0.4 Δoct Absorption spectrum: λmax = 510 nm = 243 kJ mol-1 3 d4 ions e.g. [V(OH2)6]3+ e.g. [Cr(OH2)6]3+ + 0.6 Δoct CFSE = - 0.4 Δoct = - 0.6 Δoct + 0.6 Δoct CFSE = - 0.4 Δoct = - 1.6 Δoct + P 4 Factors affecting the magnitude of Δoct High and Low spin Complexes High spin complex Low spin complex e.g. Δ is small Δ is large electrons occupy eg and t2g orbitals electrons pair in t2g oribtals before singly before pairing occupying eg orbitals [Fe(OH2)6]2+ Δoct = 10 000 cm-1 [Fe(OH2)6]3+ Δoct = 14 000 cm-1 [Co(OH2)6]2+ Δoct = 9 700 cm-1 [Co(OH2)6]3+ Δoct = 18 000 cm-1 e.g. [Co(NH3)6]3+ Δoct = 22 900 cm-1 [Rh(NH3)6]3+ Δoct = 34 100 cm-1 [Ir(NH3)6]3+ Δoct = 41 000 cm-1 5 eg eg I- < Br- < S2- < SCN- ≈ Cl-< NO3- < F- < OH- < ox2t2g < H2O < NCS- < CH3CN < NH3 ≈ en < bpy < phen ≈ NO2- < PR3 < CN- ≈ CO t2g M.J. Winter, d-block Chemistry, p.83 6 Octahedral Crystal Field ions, Oh field d-orbital energies in… free ion spherical ligand field + 0.6 ∆oct octahedral ligand field - 0.4 ∆oct x2-y2 yz z2 xz xy + 0.6 ∆oct x2-y2 yz z2 xz - 0.4 ∆oct xy the complex has a Crystal Field Stabilisation Energy (CFSE) ions, Oh field ions, Oh field High Spin eg eg + 0.6 ∆oct + 0.6 ∆oct t2g t2g - 0.4 ∆oct - 0.4 ∆oct Low Spin eg + 0.6 ∆oct t2g - 0.4 ∆oct + 0.6 ∆oct - 0.4 ∆oct 1 ions, Oh field ions, Oh field eg eg 1. What is the CFSE of [Fe(CN)6]3-? eg + 0.6 ∆oct + 0.6 ∆oct - 0.4 ∆oct t2g - 0.4 ∆oct t2g eg. 2. If the CFSE of [Co(H2O)6]2+ is -0.8 ∆oct, what spin state is it in? ions, Oh field t2g + 0.6 ∆oct Only - 0.4 ∆oct can be high or low spin Jahn-Teller Distortion configurations Jahn-Teller Distortion z z-out: d-orbitals with z-component are stabilised y Oh field x + 1/2 δ1 eg - 1/2 δ1 ∆oct + 2/3 δ2 t2g The complex ligands contract or elongate giving a - 1/3 δ2 2 Jahn-Teller Distortion Jahn-Teller Theorum z-in: d-orbitals with z-component are destabilised "For a nonlinear molecule in an electronically degenerate state distortion must occur to lower the symmetry, remove the degeneracy and lower the energy" Oh field x2-y2 + eg x2-y2 z2 1/ 2 δ1 eg x2-y2 z2 z2 - 1/2 δ1 t2g yz xz xy + 1/3 δ2 yz xz Oh z-in Na2[CuF4] Oh z-out Cr2F5 z-out xy ∆oct t2g e.g. K2[CuF4] Oh Cr(II) = high spin d4 yz xz xy - 2/3 δ2 e.g. [Cu(OH2)6]2+ Cu(II) = d9 z-out Square planar geometry: ML4 Oh z-out square planar x2-y2 eg x2-y2 x2-y2 z2 z2 xy t2g xy yz xz z2 xy yz xz yz xz d8 complexes: some Ni(II), and Pd(II), Pt(II) 3 Tetrahedral crystal field splitting Splitting of d-orbitals in a tetrahedral crystal field eg Δoct metal ion in free space in symmetrical field t2g x2-y2 yz z2 xz xy 1 Effect of crystal fields on complex geometry Spin orbit coupling is particularly significant for metals lower in the periodic table but for 1st row transition metal complexes the formula can be used in a simplified form because: “The spin contribution outweighs the orbital angular momentum contribution to μeff" Spin only magnetic moment, μSO μSO = n (n + 2) where n = number of unpaired electrons μSO = 2 S (S + 1) where S = total spin quantum number = n / 2 2 Spin only magnetic moment, μSO Measurement of magnetic susceptibility μSO = n (n + 2) where n = number of unpaired electrons Gouy Balance What is the spin only magnetic moment of …..? eg x2-y2 z2 [Co(OH2)6]2+ n= pivotal balance μSO = = yz xz t2g xy yz xz t2 xy [NiCl4]2μSO = z2 = x2-y2 [Ni(CN)4]2n= sample e x2-y2 n= scale N S magnetic field xy μSO = z2 = yz xz 3 Values of μeff for high spin Oh complexes Effective magnetic moment, μeff From experimentally measured molar magnetic susceptibility, χm T is temperature in Kelvins = eh = 4 π me 9.27 x 10-24 J T-1 0 0 0 d1 1/ 2 1.73 1.7 - 1.8 d2 1 2.83 2.8 - 3.1 d3 3/ 2 3.87 3.7 - 3.9 Ti3+ Cr3+ Cr2+, μB d0 Sc3+, Ti4+ V2+, 2.83 is the magnetogyric ratio μSO(BM) dn V3+ μeff in Bohr Magnetons: S M ion μobs (BM) Mn3+ d4 2 4.90 4.8 - 4.9 Mn2+, Fe3+ d5 5/ 2 5.92 5.7 - 6.0 Fe2+, Co3+ d6 2 4.90 5.0 - 5.6 Co2+ d7 3/ 2 3.87 4.3 - 5.2 Ni2+ d8 1 2.83 2.9 - 3.9 Cu2+ d9 1/ 2 1.73 1.9 - 2.1 Zn2+ d10 0 0 0 4 Colour in TM complexes Colour of d-d transitions depends on magnitude of Δ [Ti(OH2)6]3+ Absorption spectrum: λmax = 510 nm white light 400-800 nm 490-580 nm The Colour Wheel If red light is absorbed Blue: 400-490 nm yellow-green: 490-580 nm Red: 580-800 nm the complex appears green If purple light is absorbed the complex appears yellow wavelength, λ (nm) 5 Effect of magnitude of Δ on colour Effect of magnitude of Δ on colour 1. For a given ligand, the colour 2. For a given metal ion, the colour depends on [V(H2O)6]3+ [V(H2O)6]2+ violet light absorbed yellow light absorbed complex appears yellow complex appears violet 3+ 2+ 6 Colour and the Spectrochemical Series Weak Field Ligands Strong Field Ligands High Spin Complexes Low Spin Complexes I- < Br- < S2- < SCN- < Cl-< NO3- < F- < OH- < ox2small Δ < H2O < NCS- < CH3CN < NH3 < en < bpy large Δ < phen < NO2- < phosph < CN- < CO 7 Transition Metal Coordination Chemistry [Ti(H2O)6]3+ Absorption spectrum: λmax = 510 nm 490-580 nm Professor Sylvia Draper eg smdraper@tcd.ie WHERE HAVE WE BEEN ? Lecture 7: Magnetism and Colour Tetrahedral crystal field Magnetism Spin only magnetic moment Magnetism measurements Absorption of light d1 complexes and colour Effect of ∆ on colour WHERE ARE WE GOING ? hν t2g eg ∆o t2g Lecture 8: Colour continued, and Ligand Field Theory Selection rules Limitations of CFT LFSE and ∆ Factors affecting magnitude of ∆ 1. Oxidation state of metal e.g. 2. Position of metal in periodic table 3. Type of ligand in spectrochemical series Thermodynamic aspects of LFT 1 Intensity of colour depends on ….. The Intensity of the colour depends on Selection Rules and is measured by the absorbance Absorbance is defined by the Beer-Lambert equation: A = log10(I0/I) = ε.c.l where A = absorbance I0 = intensity of incident light; I = intensity of light after passing through the cell; ε = molar absorption coefficient; c = concentration; l = cell pathlength. Typical Spectrum: Octahedral Cr(III) complex Laporte Selection Rule 2. Spin Selection Rule At 590 nm, A = 0.138 A = εcl 0.138 = ε x 0.002M x 1 cm ε = 0.138/ 0.002 x 1 ε = 69 M-1 cm-1 Absorbance 1. Calculation of ε: 0.25 ? [Cr] = 0.002M 0.2 0.15 0.1 0.05 0 300 350 400 450 500 550 600 650 700 Wavelength (nm) Transitions allowed by the selection rules (high ε values > 1000 M-1cm-1). 2 Laporte Selection Rule Transitions between d-orbitals (g) forbidden by the Laporte selection rule Octahedral complexes Centrosymmetric: t2g and eg orbitals Selection rule lifted by molecular vibration s-orbital gerade e.g. d-orbital gerade p-orbital ungerade p-orbital to d-orbital by the Laporte selection rule d-orbital to d-orbital by the Laporte selection rule Tetrahedral complexes non-Centrosymmetric: t2 and e orbitals Tetrahedral complexes are usually more strongly coloured than analogous octahedral complexes 3 The Spin Selection Rule Assumptions made in CFT 1. The ligands are 2. Interactions are purely 1. Geometry 2. Magnetism 3. Colour Explains Limitations Does not allow for in M-L bonding Doesn't explain Order of ligands in the Spectrochemical Series Nephelauxetic Effect 4 The Spectrochemical Series The Nephelauxetic Effect Weak Field Ligands Strong Field Ligands High Spin Complexes Low Spin Complexes "there is in complexes than in the free ion" - some I- < Br- < S2- < SCN- < Cl-< NO3- < F- < OH- < ox2- < H2O < NCS- < CH3CN < NH3 < en < bpy < phen < NO2- < phosph < CN- < CO in M-L bonds – M and L share electrons - effective size of metal orbitals - electron-electron repulsion Nephelauxetic series of ligands F- < H2O < NH3 < en < [oxalate]2- < [NCS]- < Cl- < Br- < ICFT does not explain why some ligands with charges are than analogous ligands which are not charged Nephelauxetic series of metal ions Mn(II) < Ni(II) Co(II) < Mo(II) > Re (IV) < Fe(III) < Ir(III) < Co(III) < Mn(IV) 5 LFSE:the EXTRA stabilisation of complexes that occurs as a result of the splitting of orbitals in a ligand field (cf. to CFSE: it is always positive and no account of P is taken) LFSE Octahedral (always greater than tetrahedral) high/low spin options d4 to d7 only eg d1 d2 d3 d1 : LFSE 0.4 3/5∆o -2/5∆O t2g d2 : LFSE 0.4 X 2 = 0.8 d3 : LFSE 0.4 x 3 = 1.2 maximum d4 hs : LFSE 0.4 x 3 – 0.6 = 0.6 d4 d4 d5 eg LFT is identical to CFT, except that it allows for some in M-L bonding. 3/5∆o -2/5∆O t2g parameters. d5 hs : LFSE 0.4 x 3 – 0.6 x 2 = 0 minimum d5 ls : LFSE 0.4 x 5 = 2.0 highest d6 hs : LFSE 0.4 x 4 - 0.6 x 2 = 0.4 d6 LFT is based on d4 ls : LFSE 0.4 x 4 = 1.6 d5 eg t2g d6 d7 d6 ls : LFSE 0.4 x 6 = 2.4 d7 3/5∆o -2/5∆O d7 hs : LFSE 0.4 x 5 – 0.6 x 2 = 0.8 d7 ls : LFSE 0.4 x 6 – 0.6 = 1.8 d8 : LFSE 0.4 x 6 – 0.6 x 2 = 1.2 maximum ls gives higher LFSE than hs option 6 Variation in LFSE with ∆excluding P) Lattice Energies (from Born Haber cycle) For 1st row M(II) chlorides: hs, Oh Ca(II) ls d6: LFSE hs d0, d5, d10 : LFSE hs Oh d3, d8 : LFSE Ti(II) V(II) The energy Cr(II)….etc when ions come together from infinite separation to form a crystal Mn+(g) + n X-(g) MXn(s) Discrepancies in lattice energies are a result of 7 Variation in Hydration Enthalpies Enthalpy of hydration: M2+(g) + xs H2O [M(OH2)6]2+(aq) 8 Variation in Ionic Radii Ca2+ Zn2+ Sc3+ Ga3+ high spin low spin Last Lecture 9 lecture course on Coordination Chemistry Prof. Sylvia Draper high spin low spin So……. CFSE, LFE and MO theory agree ! - each one building upon and improving the other low spin: steady followed by increase from t2g6 eg1 high spin: steady followed by increase from t2g3 eg1 1 Ionisation Energies of first row TM ions, hs, Oh Irving-Williams series [M(H2O)6]2+ + [EDTA]4- [M(EDTA)]2- + 6H2O Position of equilibrium depends on d5 d6 d7 d8 d9 d10 Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > Zn2+ 2 Valance Bond Theory Tetrahedral e.g. [Zn(OH)4]2- Square Planar e.g. [Ni(CN)4]2- Ligand = Lewis base Metal = Lewis acid s, p and d orbitals give with specific geometries Number and type of M-L hybrid orbitals determines geometry of the complex Octahedral Complex e.g. [Cr(NH3)6]3+ Limitations of VB theory Assumes bonding is Does not account for Can predict wrongly Does not account for 3 Molecular Orbital (MO) theory Ligand Group Orbitals MO theory considers covalent interactions e.g. molecular hydrogen Metal valance shell orbitals = Linear Combination of Atomic Orbitals (LCAO) Ligand valance shell orbitals = LCAO approach 1s e.g. [Co(NH3)6]3+ = sigma-bonded complex 1s 1s zz 2s 2p sp3 hybrid orbitals N H H atom H2 molecule H atom H H Six sp3 hybrid ligand orbitals form a set of 4 e.g. [CoF6]3- Co3+ = 6 e- t1u* 6 x F- = 12 ea1g* Complexes with M-L π-bonding e.g. H2O,OH-, lower halides Cl-, Br-, I- Total = high spin electron density to the metal centre ligand orbital and 4p π-bond σ-bond π-bond metal orbital of electron density from filled p orbitals 4s eg* t2g 3d 6 x LGO eg π-acceptor ligands e.g. CO, N2, NO, alkenes electron density from the metal centre metal orbital and t1u O C C O ligand orbitals L Mn+ a1g ML6n+ L L L L L of electron density into emtpy π∗-antibonding orbitals 5 π-acceptor ligands: back-donation to empty ligand antibonding orbitals t1u* π-donor ligands: filled ligand orbitals and empty metal orbitals t1u* a1g* small ∆oct - weak field ligand a1g* Ligand π∗-orbitals eg* 4p large ∆oct - strong field ligand 4p Ligand π-orbitals 4s (vacant) eg* 4s (occupied) 3d 3d Ligand σ-orbitals Ligand σ-orbitals (occupied) (occupied) eg Metal d electrons (d6 example) Metal d electrons t1u (d6 example) a1g Energy Mn+ ML6n+ eg t1u a1g 6L Energy Mn+ ML6n+ 6L 6