MS PowerPoint
advertisement

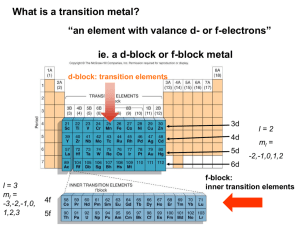
Nomenclature and Isomerism of Coordination Compounds Children's club seminar Poliraju . K Introduction Coordination chemistry Nomenclature of coordination compounds Isomerism of coordination compounds Coordination chemistry Complex Central metal ion bonded via coordinate covalent bonds to one or more molecules or ions are called ligands. Complex ion = complex with net charge. Coordination number = number of covalent bonds formed between metal ion and the ligands or ligand. Coordination chemistry Coordination compound (a) Neutral substance made up of complex ion and another ion of opposite charge. (b) Neutral complex. Ligand Lewis base – contains at one nonbonding pair of electrons. Types of complexes Cationic complexes The complex ion has a positive charge. Example: [Co(NH3)6]3+ Anionic complexes The complex carries a negative charge. Example: [Ag (CN)2] Neutral complex The complex does not carry a charge. Example: [Ni(CO)4] Double salts and coordination compounds Those which lose their identity in solution is called double salts FeSO4(NH4)2SO4. 6H2O Fe2+ + 2NH4+ + 2SO42- + 6H2O Those which retain their identity in solution is called complexes K4[Fe(CN)6] 4K+ + [Fe(CN)6]4- Who is Father of Coordination chemistry? Werner’s theory - 1893 Werner’s Theory Werner explained the nature of bonding in complexes based on the two types of valency Primary valency The number of charge on the complex ion. Secondary valency The number of ligand atoms coordinated to the metal. This is now called the coordination number. Coordination chemistry complex formation reactions Mn+ + pLx- M(L)pn-x (L = anion) Mn+ + pL M(L)pn+ ( L = molecule) Where Mn+ = Leiws acid ( center of coordination) L and Lx- = Leiws bases ( ligands) Common Ligands Monodentate ligands Contains only one donar atom Examples: H2O, NH3, CN- , SCN- , X- ( halide ions ), CO, O2Bidentate ligands Contains two donar atoms Examples: Oxalate ion (ox) C2O42-, etylenediamine (en) H2NCH2CH2NH2 Polydentate ligands Contains more than two donar atoms Example: ethylenediaminetetra acetateion (EDTA4-) Bidentate ligand ethylenediamine(en) oxalate (ox) Tridentate ligand diethylenetriamine(dien) Tetradentate ligand triethylenetetraamine(trien) Polydentate ligand (EDTA) Coordination number : 6 Nomenclature of coordination compounds : IUPAC rules Cation named before anion for ionic compounds; When naming complexes: Ligands are named first alphabetically Metal atom ion is named last Coordination state given in Roman numerals follows in parentheses Name of anionic Change -ide to Change -ite to Change -ate to ligands end with suffix -o -o -ito -ato Nomenclature : IUPAC rules Examples for anionic ligands Bromide (Br-) becomes bromo Chloride (Cl-) becomes chloro Hydroxide (OH-) becomes hydroxo Oxide (O2-) becomes oxo Nitrite (NO2-) becomes nitrito (M-O bond) or nitro (M-N bond) Carbonate (CO32-) becomes carbonato Thiosulfate (S2O32-) becomes thiosulfato Nomenclature : IUPAC rules Neutral ligands referred to by their usual names Example: ethylenediamine Exceptions Water, H2O = aqua Ammonia, NH3 = ammine Carbon monoxide, CO = carbonyl Number of each type of ligand in a complex is indicated by prefix di-.2, tri-. 3, tetra-. 4, penta-.5, If the name of the ligand begins with/ contains a prefix, bis-.2, tris-. 3, tetrakis-.4, pentakis-.5, hexakis-.6 Nomenclature : IUPAC rules If complex is an anion, its name ends with –ate appended to either the English or Latin name of the metal Example: Scandium, Sc = scandate Titanium, Ti = titanate Venadium, V = vanadate Manganese, Mn = manganate Zinc , Zn = zincate Naming coordination compounds Example 1: [Co (NH3)6]Cl3 i.e . Co3+ Complex ion = hexaamminecobalt() Counter ion = chloride [Co (NH3)6]Cl3 = hexaamminecobalt()chloride Formula of coordination compound Name of the coordination compound K3[Fe(CN)6] Potassium hexacyanoferrate() [CoCl(NH3)5]Cl Pentaamminechlorocobalt()chloride Na[PtBrCl(NO2)(NH3)] Sodium amminebromochloronitrito-Nplatinate() K2[Ni(CN)4] Potassium tetracyanonickelate() Fe4[Fe(CN)6] Iron() hexacyanoferrate() Metals and chelates in living system The heme unit in hemoglobin involves a rigid chelating ligand, H2O or O2 or CO can be the 6th ligand. Metals and chelates in living system Chlorophyll, involved in photosynthesis, is a complex ion of Magnesium(II)ion. Types of Isomerism 1. Structural Isomerism a) Coordination isomerism The interchange of ligands between cationic and anionic entities of different metal ions present in a complex. Example : Co(NH3)ClBr Co(NH3)BrCl and [Co(NH3)6][Cr(CN)6] + + - Br NH3 NH3 NH3 NH3 Co NH3 Co Cl NH3 NH3 NH3 NH3 Br NH3 Cl- b) Ionization isomerism The exchange of groups between the complex ion and the ions outside the complex Example: Pt (NH3)4Cl2Br2 and Pt(NH3)4Br2Cl2 produce different ions in solution C) Hydrate isomerism : It occurs when water forms a part of the coordination entity or is outside it Cr(H2O)6Cl3 Cr(H2O)5ClCl2.H2O Cr(H2O)4Cl2Cl.2H2O violet green dark green (three ionic chlorines) (two ionic chlorines) (one ionic chlorine) d) Linkage isomerism Linkage isomerism arises in a coordination compound containing ambidentate ligand. Example: [Co(NH3)5(NO2)]Cl2 O O N NH3 O 2+ NH3 Co 2+ N Cl2- O NH3 Cl2NH3 Co NH3 NH3 NH3 NH3 NH3 [Co(NH3)5(NO2)]Cl2 – (Red) NH3 Co(NH3)5(ONO)]Cl2- (Yellow) 2. Stereoisomerism a) Geometrical isomerism: cis and trans Square planar complex: Example : Ma2b2 [Pt(NH3)2Cl2] Mabcd [PtBrClNH3(py)] Geometrical isomerism in Octahedral complex Ma4b2 type (cis and trans isomers) OH2 Cl OH2 OH2 Cl Fe Fe Cl OH2 MAA2b2 type - Three isomers ( two cis and one trans) trans- [CoCl2(en)2] Cl OH2 OH2 cis-[CoCl2(en)2] OH2 OH2 Geometrical isomerism in Octahedral complex Ma3b3 type [Co(NH3 )3(NO2 )3 b) Optical isomerism Optical isomers are mirror images that cannot be superimposed on one another. The molecules or ions that cannot be superimposed are called chiral. Optical Isomerism 20_446 Have opposite effects on plane-polarized light (no superimposable mirror images) Polarizing filter Tube containing sample Unpolarized light Polarized light Rotated polarized light 20_448 Mirror image of right hand Left hand Right hand 20_450 Cl N N Co N N N N Co N Co N cis N N N Isomer II cannot be superimposed exactly on isomer I. They are not identical structures. Cl Cl Cl N Cl (a) Cl N Cl trans The trans isomer and its mirror image are identical. They are not isomers of each other. Co Cl N Cl N N N N Co Cl Isomer I N Isomer II N (b) Isomer II has the same structure as the mirror image of isomer I. Optical Isomerism The absolute configuration be designated Lambda Λ (left-handed) and Delta Δ (right-handed) cis-isomers of octahedral complexes with 2 bidentate ligands and 2 monodentate ligands Example : cis-[CoCl2(en)2] complex (cis-bis chel.ates) Optical Isomerism Octahedral complexes with 3 bidentate ligands Example K3[Cr(C2O4)3] complex (tris chelate)