Chemistry Lab Notebook Format - High School Guide
advertisement

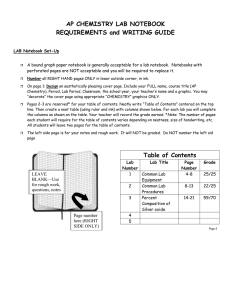
Kapuskasing District High School CHEMISTRY LAB NOTEBOOK FORMAT LAB NOTEBOOK A lab notebook is simply a record of an experiment as it is performed. Your lab notebook should contain enough information about the experiment so that later you, or someone else, can understand what has been done without referring to the lab manual. In your lab notebook, include A table of contents (with the title of the lab, the pages, and the date of the experiment). This will be updated for each experiment Number the pages in your notebook Important Reminders for a Lab Report 1) Spelling 2) Avoid personal pronouns 3) Headings should stand out 4) Neatness counts - use rulers when needed (especially when using tables and graphs) PRELAB Before coming to the lab each student must be prepared. It is expected that each student has read the lab, copied and read the MSDS safety precautions for the chemicals being used. The MSDS must be submitted by the students on the day of the experiment. It will be signed and dated by the teacher before the lab is completed. LAB REPORT Data The data section of your report should have a title, date, and include the name of you lab partner. All data should be recorded directly into your lab book as the experiment is being performed. Whenever possible, data should be recorded in tables. Each table should have a title and column headings and units. If a mistake is made in entering data, draw one or two lines through the error(s) and record the correct data. DO NOT erase or white out errors. The data recorded for each experiment should be accurate, detailed, and concise. Purpose The purpose of your experiment consists of one or two sentences describing what is being tested, investigated or measured. DO NOT copy the purpose directly from your lab. Materials List the materials used in the experiment, include the amount of each chemical used. Safety Discuss the hazards potentially posed by the chemicals used in the lab, and suggest safety measure that will be taken to prevent these problems ( handling and disposal). Theory and Procedure In general, a fairly detailed theory and procedure is found in the lab. DO NOT copy this into your report. State “As per lab ’Name and Textbook, page number”. Briefly discuss any theory or procedure that was different than outlined in the lab. Analysis and Results Briefly describe the analysis performed to accomplish the purpose of the experiment. It may be convenient to separate this section into parts corresponding to your lab. In each part, you may be required to show sample calculations for EACH type of calculation performed in your experiment. The results of calculations may be stated as equations, placed in tables, or in additional columns of your data table, if applicable. Indicate where all graphs and the corresponding data tables may be found. Graphs should be staples/taped into the notebook. Be sure all graphs have descriptive titles and clearly labeled axes. Show all slope calculations directly on your graph. Summarize the main findings from the graph in your results section. Show numerical comparisons to accepted values; for example percent difference or percent deviation (See Physics 12 McGraw-Hill Ryerson page 607). Answer any questions found in the lab. Conclusion A concluding discussion of the implications of your results is included in this section. 1. Discuss qualitative and quantitative results in relation to the expected behaviour or theory and try to explain the discrepancies. (Summarize the results) 2. Discuss the precision and accuracy of your results and any possible sources of error. (See Physics 12 McGraw-Hill Ryerson page 606-607) 3. Include suggestions concerning improvements to the experiment. Adapted from Experimental Investigations on Introductory Physics, Department of Physics, Lakehead University 2001