Flame Test Lab
advertisement

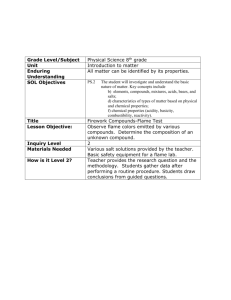
Flame Test Lab Group name: The Dough Boys B. Silly Lab Partners N.O. Kidding I.M. Goofy O. Really Introduction The first spectroscopes’ invention is accredited to Gustav Robert Georg Kirchoff and Robert Wilhem Bunsen. Early spectroscopes were prisms with graduations marking off the wavelengths of light. Later spectroscopes worked by allowing light through a slit into a collimating lens that transforms the light in to a thin beam of parallel rays. The light then passes through a prism that refracts the beam into a spectrum. With the spectroscope, we can measure properties of light like wavelength and intensity. Using the equation E = hc/λ, one can find the energy of the spectra or emissions lines created and measured by the spectroscope. One can indentify a metal by its unique emissions spectrum produced when heated in a flame. This is caused by electrons without sufficient energy remain in a higher energy level falling back to a lower one emitting energy as a photon of light. In this lab, seven metal salt compounds are put in a flame to observe and measure their emissions spectra. The emissions spectra will be observed and the emission lines’ energy will be calculated using the wavelengths observed through a spectroscope. Experimental Seven different metal salt compounds—KCl, SrCl2, CaCl2, LiCl, NaCl, CuCl2 and BaCl2—were mixed with distilled water to create a paste. The compounds are placed in a number twelve well tray and carefully kept track of. About the tip of a scoopula full of each compound is used. The Bunsen burner is then lighted and trimmed. J The nichrome wire is cleaned by putting it in the flame and dunking it in a beaker full of distilled water. The wire is considered sufficiently clean when the wire in the flame emits no color when first placed into the flame. The wire is then dipped into the first well containing the first metal salt compound. The wire now covered in the compound is placed in the flame and the flame color is recorded. The flame is viewed through the spectroscope to see the emissions spectra. The flame color and the two most prominent emission lines’ wavelengths are recorded the wire is then cleaned using the same process as before and the compound in the next well is put into the flame until the process is completed for all of the compounds 2 Results and Discussion The following table items are the results and calculations from the experiment. Wavelength Compound Flame color Line 1 (nm) Line 2 (nm) Energy Line 1 (J) Line 2 (J) -19 4.4 x 10-19 KCl Purple-pink 600 450 3.3 x 10 SrCl2 Dark red 620 680 3.2 x 10-19 2.9 x 10-19 CaCl2 Orange 600 570 3.3 x 10-19 3.5 x 10-19 LiCl Fuchsia 670 590 3.0 x 10-19 3.4 x 10-19 NaCl Yellow 600 550 3.3 x 10-19 3.6 x 10-19 CuCl2 Green 600 560 3.3 x 10-19 3.5 x 10-19 BaCl2 Yellow-green 590 550 3.4 x 10-19 3.6 x 10-19 The colors of the flame are caused by electrons that when heated become excited and move to a higher energy level. When the electrons no longer have the energy to stay in that energy level, they fall back to a lower energy level. The excess energy from the differences in the energy levels is released in the form of a photon of light. The different colors are created because the photon’s energy is based on how much energy the falling electrons release. The electrons that fall from higher energy levels release more energy than an electron that falls from a lower energy level. The more energy released the closer the light is to violet in the spectrum. A lower energy release is closer to the red side of the spectrum. The elements that are farther down the periodic table seem to have emissions closer to the violet end of the spectrum while the elements higher up in the periodic table have emissions closer to the red end of the spectrum. This is somewhat unexpected. The elements higher up on the periodic table have fewer electrons and so are more tightly held by the nucleus. Because they are more tightly held, it should require more energy to excite and electron, which means more energy would be given off when that electron returns to a lower energy level. The only truly green flame was produced by the only transition metal cation used in this experiment. This suggests that the presence of electrons in the d-orbitals affects the observed color of the flame. 3 There are many sources of possible error in this lab. The same wire was used for each compound which means that there is the possibility that residue from the previous compound could have been present in the next burning. This would cause an inaccurate reading with the spectrometer and therefore and inaccurate energy calculation. Another error could come from the fact that certain compounds had to be held to the Bunsen burner flame in a very specific manner in order to get the correct flame color. If the compounds were held incorrectly, the data would be wrong. Another area where we could have accrued error is in the reading of the spectrometer. The emissions sometimes lasted for only a few short seconds and so an accurate reading was harder to get. In general, imprecise tools and inconsistent procedures can account for most of the error in this lab. Conclusion The energy of metal salt compounds’’ emission lines when heated were examined in this lab. It was found that elements that released the highest energy photons tended to be closer to the bottom of the periodic table. For example, lithium (atomic number 3) had emission lines of 670 nm and 590 nm while barium (atomic number 56) had significantly shorter wavelengths of 590 nm and 550 nm. Peer evaluation N.O. Kidding: 2 (note: he spent too much time goofing around) I.M. Goofy: 5 O. Really: 4 Sample calculations KCl line 1 energy: E = hc/λ E = (6.626 x 10-34 Js)(3 x 108 m/s)/(6.00 x 10-7 m) = 3.3 x 10-19 J 4