Bell's Palsy October 2012 - University of Texas Medical Branch
advertisement

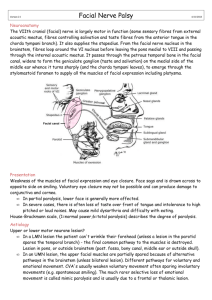
Bell's Palsy October 2012 TITLE: Bell’s Palsy SOURCE: Grand Rounds Presentation, Department of Otolaryngology The University of Texas Medical Branch (UTMB Health) DATE: October 29, 2012 RESIDENT PHYSICIAN: Viet Pham, MD FACULTY PHYSICIAN: Dayton Young, MD FACULTY PHYSICIAN: Tomoko Makishima, MD, PhD SERIES EDITOR: Francis B. Quinn, Jr., MD ARCHIVIST: Melinda Stoner Quinn, MSICS "This material was prepared by resident physicians in partial fulfillment of educational requirements established for the Postgraduate Training Program of the UTMB Department of Otolaryngology/Head and Neck Surgery and was not intended for clinical use in its present form. It was prepared for the purpose of stimulating group discussion in a conference setting. No warranties, either express or implied, are made with respect to its accuracy, completeness, or timeliness. The material does not necessarily reflect the current or past opinions of members of the UTMB faculty and should not be used for purposes of diagnosis or treatment without consulting appropriate literature sources and informed professional opinion." INTRODUCTION First reported by Sir Charles Bell in 1818, Bell’s palsy describes a facial paralysis with few associated symptoms that is rapid in onset and generally followed by spontaneous resolution. Despite being the most common diagnosis of facial nerve paralysis, Bell’s palsy is actually a diagnosis of exclusion and can induce a great deal of patient anxiety with its socially devastating sequelae. Historically thought to be an idiopathic process, mounting evidence conveys a significant role of the herpes simplex virus (HSV) in the pathogenesis of this condition, and consequently, has imparted an importance influence on the direction of viable treatment options. FACIAL NERVE ANATOMY The facial nerve is a large structure containing between 7,000-10,000 fibers. In addition to its primary role in controlling somatic facial musculature movement, the facial nerve also generates contributions to the sensation of taste with fibers from the nucleus tractus solitarius and to parasympathetic secretomotor function with fibers from the superior salivatory nucleus. The nerve can be divided into specific segments as it emerges from its origin in the brainstem and courses through the temporal bone and out the stylomastoid foramen. Sometimes referred to as the cisternal segment, the intracranial portion of the facial nerve refers to the fibers as it leaves the motor nucleus in the brainstem, wraps around the abducens nucleus, and exits out the ventral pons just medial to the spinal trigeminal nucleus. Axons responsible for the parasympathetic and sensory functions of the facial nerve comprise the nervus intermedius. Of note, the dorsal portion of the motor nucleus receives bilateral cortical input while the ventral aspect of the nucleus only receives unilateral input from the contralateral cortex. Consequently, control of the upper facial muscles is regulated by both brain cortices, allowing a patient to demonstrate some upper movement despite a central lesion. Conversely, a peripheral lesion will involve all of the facial muscle groups on the ipsilateral side. 1 Bell's Palsy October 2012 After exiting the brainstem, the facial nerve enters the internal auditory canal (IAC) and represents the meatal segment. At an average length of 8mm and without releasing any peripheral branches, the facial nerve and the nervus intermedius are separated from the vestibulocochlear nerve by the falciform crest transversely and Bill’s Bar vertically to traverse in the anterosuperior aspect of the IAC more distally. The labyrinthine segment denotes where the facial nerve runs 3-4mm from the IAC to the geniculate ganglion and gives off branches to the greater superficial petrosal, lesser petrosal, and external petrosal nerves. The greater superficial petrosal nerve arises from the nervus intermedius and receives a branch from the tympanic plexus as it carries parasympathetic fibers toward the pterygopalatine ganglion along with taste fibers from the palate. It is joined by the deep petrosal nerve from the internal carotid sympathetic plexus along the way to comprise the Vidian nerve and regulate lacrimation. The lesser petrosal nerve serves a similar role as it traverses past the tympanic plexus and synapses in the otic ganglion to deliver parasympathetic fibers to the parotid gland via the auriculotemporal nerve. As the facial nerve crosses between the cochlea and vestibule from the geniculate ganglion, it takes a more horizontal orientation for 8-11mm as it goes posteriorly toward the pyramidal eminence as the tympanic segment, sometimes aptly designated as the horizontal segment. The mastoid segment signifies where the nerve travels 8-14mm vertically from the pyramidal eminence toward the stylomastoid foramen in the temporal bone, and hence, it is occasionally referred to as the vertical segment. Here, the facial nerve provides branches to the stapedius muscle, and it sends parasympathetic innervations to the submandibular and sublingual glands via the submandibular ganglion by way of the chorda tympani. The chorda tympani also delivers taste fibers to the anterior two-thirds of the tongue. The posterior auricular nerve arises from the facial nerve close to the stylomastoid foramen to join the auricular branch of the vagus nerve to eventually innervate the intrinsic auricular muscles and the occipitalis. After leaving the stylomastoid foramen, the facial nerve travels approximately 15-20mm before dividing into the upper and lower major divisions at the pes anserinus, where it will then be distributed among the temporal, zygomatic, buccal, marginal, and cervical branches. HOUSE-BRACKMANN SCALE The most commonly utilized classification schema to describe the severity of any facial palsy is the House-Brackmann (HB) scale first introduced in 1985 and has served to help convey among physicians the degree of facial paralysis using a universal system. Table 1 below presents a visual adaptation of this system. 2 Bell's Palsy October 2012 Table 1. House-Brackmann Scale DEMOGRAPHICS Bell’s palsy is felt to carry an incidence of approximately 30 out of 100,000 individuals, although many have noted a higher rate in pregnant females (3.3. times greater) and diabetic patients (4-5 times greater). Both genders tend to be affected equally around middle age, but young females between 10-19 years of age and males older than 40 years of age have been noted to be affected more often (2 times and 1.5 times greater, respectively). The right and left facial nerves are equally involved, but simultaneous bilateral involvement is a rare event with a 1% occurrence. Recurrence occurs 10% of the time, and only 10% of affected individuals report a positive family history of similar symptoms. NATURAL HISTORY Peiterson provided an insightful investigation into the natural course and spontaneous recovery rate of Bell’s palsy while monitoring the outcomes of more than 1,000 patients over a fifteen-year period who did not receive treatment for their facial paresis (Peiterson 1985). The mean age of onset was between 40-44 years, but it was less common in those younger than 15 years and those older than 60 years of age. There was no gender predilection, and a recurrence rate between 6-9% was observed in this population. Some associated symptoms included a reduced stapedial reflex, phonophobia, postauricular pain, dysgeusia, and decreased lacrimation. Approximately 31% experienced a facial paresis with complete recovery occurring in 95% of these patients. The remaining 69% presented with a complete facial paralysis, but some recovery by three weeks after onset was noted in 85% of these patients. A satisfactory outcome, referring to a recovery of facial function to a HB scale 1 or 2, was observed in 84% of these individuals (HB 1 in 71% and HB 2 in 13%). Complete recovery was observed by one month in 85%, but the other 15% progressed to complete degeneration with some signs of recovery manifesting between 3-6 months afterward. These patients were noted to have a higher incidence of sequelae such as diminished facial function, contractures, synkinesis, or tearing. 3 Bell's Palsy October 2012 PATHOPHYSIOLOGY While originally felt to be an idiopathic process, two theories arose in an attempt to explain the underlying process leading to Bell’s palsy. McGovern postulated autonomic vascular instability, possibly triggered by cold temperature or psychosomatically, resulted in spasm of arterioles nourishing the facial nerve (McGovern 1955). The suffocation of nutrients would induce a secondary ischemia and incite edema of the nerve, and consequently, lead to compression against the fallopian canal and engender a facial palsy. Antoni coined the term acute infectious polyneuritis cerebralis acusticofacialis in 1919 to suggest an infectious etiology to Bell’s palsy (Friedman 2000). Edema of the facial nerve was also fundamental to the pathophysiologic process, but it was believed that this was related to an inflammatory response to a viral presence. HSV was first proposed as the responsible agent by McCormick in 1972, but it would be over twenty years later for other investigations to lend credence to this line of thinking. Burgess reported the presence of HSV type 1 (HSV-1) DNA in the temporal bone of a patient who passed away six days after developing Bell’s palsy (Burgess 1994). Mice inoculated with HSV-1 DNA in either the tongue or auricle exhibited a transient facial paresis approximately 6-9 days after inoculation with spontaneous recovery 3-7 days afterward. Neural edema was noted on histological analysis with vacuolar degeneration and infiltration of inflammatory cells. HSV antigens were encountered in the facial nerve, geniculate ganglion, and facial nerve nucleus 6-20 days after inoculation (Surgita 1995). Murakami evaluated the endoneural fluid of patients undergoing transmastoid decompression during the active phase of facial palsy secondary to a myriad of etiologies and found HSV-1 in 11 out of 14 patients with Bell’s palsy, but no signs of varicella-zoster virus (VZV) or Epstein Barr virus were appreciated. Conversely, VZV but not HSV-1 was noted in those with Ramsay Hunt syndrome while both viruses were not present in those with a facial palsy from trauma or neoplasm (Murakami 1996). Furuta employed polymerase chain reaction to detect HSV-1 in the saliva of patients with either Bell’s palsy or Ramsay Hunt and compared it to a control group of people seropositive for HSV but without a facial paresis. HSV-1 was found in 50% of individuals with Bell’s palsy compared to 19% of the control group. Furthermore, the timing of testing appeared to play a significant role as 40% of those with Bell’s palsy and 7% of those with Ramsay Hunt expressed HSV-1 when tested within 7 days of facial palsy before it became essentially undetectable by the second week (Furuta 1998). Lymphocytic infiltrate, myelin degeneration (McKeever 1987), and perineural edema (Donoghue 1983, Podvinec 1984) have been histologically noted in Bell’s palsy, which is especially prominent in the labyrinthine segment of the facial nerve. This is accompanied by reports of a conduction block at this site (Gantz 1982) to suggest that the nerve gets entrapped at the meatal foramen (Fisch 1983), the narrowest portion of the fallopian canal. Increased or prolonged constriction results in ischemia and subsequent Wallerian degeneration of the nerve. 4 Bell's Palsy October 2012 PHYSICAL EXAMINATION A thorough medical history and comprehensive physical examination is paramount to approaching a patient with suspected Bell’s palsy. While it may not necessarily add a significant amount of additional information if the abnormality is indeed related to Bell’s palsy, it can exclude other conditions that may masquerade with a similar presentation. Typically, Bell’s palsy occurs over a 24-48 hour span that can progress to complete paralysis over 1-7 days. A neoplasm, such as a vestibular schwannoma, should be ruled out if evolution progresses past three weeks. Aside from an absent ipsilateral acoustic reflex, patient should present with a symmetrical audiological function, and a diagnosis of Bell’s palsy should be questioned if there are vertiginous complaints. Keeping cognizant of the contralateral upper motor neuron contribution to upper facial muscle movement, there should be a high suspicion for a cerebrovascular accident or central neurological lesion if the facial palsy does not encompass all of the facial nerve branches. Chronic otitis media or cholesteatoma should be considered if there is concurrent ear disease, while a vesicular eruption will warrant concern for Ramsay Hunt syndrome. Although a small proportion of Bell’s palsy patients may present with bilateral weakness, systemic neurological disorders with associated facial paralysis should be assessed such as Guillain-Barre syndrome or Lyme disease. Individuals with type II neurofibromatosis may demonstrate bilateral facial paralysis secondary to bilateral vestibular schwannomas. While regarded as a rare situation that can occur with Bell’s palsy, facial palsy recurrence often argues against the diagnosis and more to other conditions such as Melkersson-Rosenthal syndrome. RADIOLOGY Generally, the rationale behind radiographical imaging in the midst of Bell’s palsy is the aspiration that it might help localize a lesion to attribute the facial palsy. Computed tomography is better utilized to rule out other causes of facial paralysis including temporal bone fractures, mastoiditis, or cholesteatoma. On the other hand, magnetic resonance imaging’s (MRI) superiority in soft tissue delineation may identify an area of enhancement along the course of the facial nerve, but there has been no significant correlation between the site or degree of nerve enhancement and the clinical outcome. Ultimately, MRI is more commonly utilized to exclude neoplasm such as vestibular schwannoma. TOPOGRAPHY Some have advocated topographical testing on the premise that a lesion can be localized based on abnormalities that may affect the function of certain branches of the facial nerve. For example, decreased lacrimation would manifest with an abnormal Schirmer test to suggest a lesion near or involving the greater superficial petrosal nerve. Similarly, an absent stapedial reflex would imply involvement of the stapedial branch of the facial nerve while electrogustometry and salivary flow studies would evaluate the chorda tympani. However, these endeavors have been unable to consistently predict the location of a lesion nor the resulting clinical outcome. 5 Bell's Palsy October 2012 AUDIOLOGY As alluded above, individuals with Bell’s palsy typically exhibit symmetrical audiometric function with the one exception that there may be an absence to the acoustic reflex ipsilateral to the side of the paralysis. Similar to the reasoning with radiographical imaging, audiological evaluation is employed more to exclude retrocochlear pathology which typically manifest more with asymmetrical thresholds and decay, but not necessarily absence, to the acoustic reflex. ELECTROPHYSIOLOGY Sunderland’s classification describes five degrees of nerve injury. Neuropraxia is the first degree and designates a localized conduction block but with structural continuity of the axon so that Wallerian degeneration does not occur. Axonotmesis is the second degree of injury and describes axoplasmic disruption but with endoneural sheath preservation. Unlike neuropraxia, Wallerian degeneration does arise from this interrupted axonic integrity. Neurotmesis groups together the third, fourth, and fifth degrees of nerve injury with subdivisions depending on the status of the perineurium and epineurium. This disruption of the axonal and supportive cells incites a more rapid Wallerian degeneration compared to axonotmesis and is associated with a poorer and less predictable outcome. Electrophysiologic tests draw upon these concepts and perhaps provide the most important diagnostic tools in evaluating patients with Bell’s palsy from the prognostic information they engender. These tests are not initiated in those who present with only a paresis; rather, they are best employed three days after progression to a complete paralysis as Wallerian degeneration may not occur early in the clinical course. Furthermore, assessing for objective prognostic information in those with a partial facial paralysis may be superfluous in light of the favorable prognosis for spontaneous recovery as outlined by Peiterson (Peiterson 1985). Nerve excitability testing (NET) was first described by Hilger in 1964 and compares the electrical thresholds required to elicit minimal voluntary muscle contraction between the normal side and the paralyzed side of the face. A difference of 3.5mA is considered indicative of severe degeneration and predictive of an unfavorable outcome. Inaccurate within the first three days of the onset of complete paralysis, NET is dependent on subjective comparison and potentially susceptible to inter-observer variability. Maximum stimulation testing (MST) is similar to NET with the distinction that it compares muscle contraction with maximum stimulation which can be uncomfortable for patients. A greater degree of muscle weakness is concordant with worsening degeneration, and like NET, MST is also not useful within the first three days of paralysis and subject to observer assessment. Electroneuronography (ENoG) is another comparative electrophysiologic assessment but has the advantage of being observer independent as a quantitative analysis. The evaluation entails stimulating the facial nerve at the stylomastoid foramen and measuring the muscular response near the nasolabial groove. The resulting action potential for both sides are tested and presented as a percentage of degeneration comparing the affected side with the normal side of the face. As with NET and MST, ENoG is inaccurate within the first three days of complete paralysis. 6 Bell's Palsy October 2012 The prognostic value of ENoG was elucidated by Esslen when full recovery was noted in 88% of patients who demonstrated less than 90% degeneration on ENoG. This percentage decreased significantly to 30% if there was between 90-95% degeneration, and full recovery was not observed in those with complete degeneration (Esslen 1977). Fisch expanded on this with the assertion that satisfactory spontaneous recovery was highly likely if there was less than 90% degeneration on ENoG obtained within three weeks of paralysis onset. Patients who exhibited 90% degeneration had a higher likelihood of progressing to 95% degeneration, which is important in light of the conclusion that a permanent unsatisfactory result, denoted as a residual facial palsy of a HB scale of 3 or worse, was present in 50% of individuals with 95-100% degeneration by two weeks of paralysis onset (Fisch 1981). Electromyography (EMG) is a quantitative test that measures action potentials produced with volitional muscle movement. Normal contraction is signaled by diphasic or triphasic waveforms, fibrillation connotes degeneration, and polyphasic potentials indicate reinnervation. EMG is most valuable as a complementary test to accompany ENoG as it can better ascertain if a facial palsy is likely undergoing degeneration or regeneration. Because regenerating nerve fibers do not complete a summation potential on ENoG, the presence of defibrillation and motor unit potentials on EMG would suggest regeneration. Degeneration would be favored if there was only fibrillation potentials and no voluntary motor units. An important caveat to EMG in the setting of degeneration is that fibrillation will not be encountered until approximately 10-14 days after onset of paralysis. MEDICAL MANAGEMENT Regardless of the degree of facial palsy present, the eye needs to be evaluated and managed to avoid disastrous ocular-related consequences that will manifest if incomplete eye closure is present such as a corneal abrasion or exposure keratitis. Especially in paretic cases, expectant management may be an acceptable treatment modality if the patient is closely followed for spontaneous recovery. Numerous studies have investigated the role of steroids in addressing Bell’s palsy, typically with regimens such as prednisone 1mg/kg/d up to 70-80mg. This is commonly tapered after 5-7 days, although treatment may be extended if no improvement is appreciated. Many investigations have cited benefit to steroids (Adour 1972, Katusic 1986), especially if they are implemented early in the course of the disease (Brown 1982, Williamson 1996). Shafshak echoed the importance of prompt intervention, recommending beginning prednisolone within 24 hours of paralysis (Shafshak 1994). Austin evaluated prednisone in a randomized, double-blinded, placebo-controlled trial and concluded that there was improved recovery with the medication. A statistically insignificant trend for denervation prevention was also observed (Austin 1993). This study and two others were included in a meta-analysis of 27 prospective and 20 retrospective trials investigating the benefit of steroids. These three studies qualified for the meta-analysis inclusion criteria seeking prospective, controlled trials utilizing at least 400mg of prednisone started within seven days of paralysis and concluded that steroids improved complete recovery by 17%. There was a generally positive benefit observed in the excluded trials with an acquisition of complete recovery ranging between 49-97% using steroids and 23-64% without (Ramsey 2000). The 7 Bell's Palsy October 2012 latest Cochrane Review at the time of this manuscript concurs with this sentiment that steroids increase the frequency of complete recovery (Salinas 2010). There are reports that contradict these findings (May 1976, Stankiewitz 1987), and a literature review of nine studies over a 45 year-span comparing steroids to placebo did not note a difference in rate of recovery or synkinesis. Although acknowledging that most of these investigations were underpowered and that there was a beneficial trend observed in some of the studies, the mild side effect profile and potentially positive therapeutic effect to steroids led to the conclusion that there was “probable” benefit with steroids (Grogan 2001). Controversy arises with the use of steroids in the pediatric population with Bell’s palsy as no benefit was observed in children (Prescott 1987). A literature review of nine reports over a thirty year-period could not definitively support the role of steroids in this demographic. Only one of the studies specifically targeted children, and no statistical sub-analysis was conducted on this population in the other trials. Although some benefit was observed in four of the investigations, heterogeneity among them all precluded a meta-analysis or establishing a formal recommendation (Salman 2001). Antivirals were evaluated as an adjuvant therapy to steroids in a double-blind trial combining prednisone with either acyclovir or placebo initiated within three days of paralysis onset. The combination of prednisone and acyclovir resulted in less facial weakness on MST and a lower rate of unsatisfactory recovery (Adour 1996). As individual treatment modalities, however, prednisone was felt to be more beneficial than acyclovir (De Diego 1998), and a subsequent literature review cited a paucity of studies to assert a definite therapeutic benefit but supported the notion of a “possible” benefit with adding acyclovir to prednisone given the generally well-tolerated side effect profile to antiviral medications (Grogan 2001). Similar positive observations were encountered with valacyclovir (Axelsson 2003, Hato 2007), and the latest Cochrane Review at the time of this manuscript concluded that antivirals were beneficial when added to steroids but not as a single treatment endeavor apart from steroids (Lockhart 2009). SURGICAL MANAGEMENT Balance and Duel first postulated the utility of decompressing the facial nerve in 1932 for surgical treatment in Bell’s palsy. The focus of intervention localized initially to the stylomastoid foramen in the 1930’s before transitioning more toward the tympanic segment in the 1960’s. Decompression was felt to yield benefit in some accounts (Giancarlo 1970), but this was refuted in others, with the description of no improvement with decompression from the geniculate ganglion down toward the stylomastoid foramen (McNeill 1974). May had originally championed a transmastoid approach to decompress the facial nerve from the geniculate ganglion to the labyrinthine segment but without involving the meatal foramen (May 1979). This ideology was later recanted as no palpable benefit was observed even when decompression was undertaken within 14 days (May 1984) nor with decompressing the mastoid segment alone (May 1985). A conduction block proximal to the geniculate ganglion was encountered during total nerve decompression via a middle cranial fossa and transmastoid approach described by Fisch 8 Bell's Palsy October 2012 (Fisch 1972). This was further elaborated upon with the report of benefit to surgery if the meatal foramen was decompressed within three weeks of paralysis onset when 90% degeneration was noted on ENoG (Fisch 1981). Gantz supported this philosophy but shortened the timeframe by which to intervene down to two weeks (Gantz 1999). In this multicenter study, Gantz compared clinical outcomes between surgery and steroids dependent on the amount of degeneration measured on ENoG. High-dose steroids without antivirals were administered if there was less than 90% degeneration, but decompression from the IAC through the tympanic segment via a middle cranial fossa approach was offered if degeneration exceeded 90% with no EMG activity by two weeks. Patients who underwent decompression past two weeks constituted a “surgical control,” while those who deferred surgery were addressed with steroids. Similar to past trials, a favorable outcome, designated as recovery to a HB I or II, was observed in patients with less than 90% degeneration on ENoG. Furthermore, patients who were decompressed within two weeks experienced a higher rate of HB I or II recovery compared to those who received steroids (91% vs. 42%) and a lower rate of unfavorable outcomes to HB III or IV recovery (9% vs. 58%). There were similar results between the surgical control and steroid management groups to insinuate the importance of intervening within two weeks of paralysis (Gantz 1999). Although the exact protocol may vary among institutions, most physicians have adopted a similar treatment scheme as outlined by Brackmann in Figure 1 below. Figure 1. Bell’s Palsy Treatment Algorithm (Brackmann 2010) 9 Bell's Palsy October 2012 CONCLUSION Bell’s palsy is a diagnosis of exclusion but remains the most common diagnosis of facial paralysis. Electrophysiological testing affords important prognostic information which may significantly impact the treatment plan and promote appropriate patient counseling. HSV is regarded as a significant factor in the pathogenesis of this condition and drives the rationale behind treatment options employed to manage affected individuals. While there is general consensus to the utility of steroid therapy, with or without antiviral treatment, spirited debate and controversy continues to shroud the role of surgical decompression. Given the current evidence, a multimodality approach may ultimately be warranted in select patients. REFERENCES Adour KK, Ruboyianes JM, Von Doersten PG, et al. Bell’s palsy treatment with acyclovir and prednisone compared with prednisone alone: a double blind, randomized controlled trial. Ann Otol Rhinol Laryngol 1996; 105:371-378. Adour KK, Wingerd J, Bell DN, et al. Prednisone treatment for idiopathic facial paralysis (Bell’s palsy). N Engl J Med 1972; 287:1268-1272. Austin JR, Peskind SP, Austin SG, et al. Idiopathic facial nerve paralysis: a randomized double blind controlled study of placebo versus prednisone. Laryngoscope 1993; 103:1326-1333. Axelsson S, Lindberg S, Stjernquist-Desatnik A. Outcome of treatment with valacyclovir and prednisone in patients with Bell’s palsy. Ann Otol Rhinol Laryngol 2003; 112:197-201. Brackmann DE, Shelton C, Arriaga MA. Otologic Surgery, 3rd Ed. Philadelphia: Saunders, 2010. pp 336-338. Briggs RD. Evaluation and Management of Bell’s Palsy. UTMB Department of Otolaryngology Grand Rounds 2002. Accessed 17 Sep 2012 <http://www.utmb.edu/otoref/grnds/Bells-Palsy2002-01/Bells-Palsy-2002-01.htm>. Brown JS. Bell’s palsy: A 5 year review of 174 consecutive cases: An attempted double blind study. Laryngoscope 1982; 92:1369-1373. Burgess RC, Michaels L, Bale JF. Polymerase chain reaction amplification of herpes simplex viral DNA from the geniculate ganglion of a patient with Bell’s palsy. Ann Otol Rhinol Laryngol 1994; 103:775-779. De Diego J I, Prim MP, De Sarriá MJ, et al. Idiopathic facial paralysis: A randomized, prospective, and controlled study using single-dose prednisone versus acyclovir three times daily. Laryngoscope 1998; 108(4 Pt 1):573-575. Dixon A, Gaillard F, et al. Facial Nerve (CN VII). Radiopaedia.org. Accessed 17 Sep 2012 <http://radiopaedia.org/articles/facial_nerve_(cn_vii)>. Donoghue O. Histopathologic aspects of Bell’s palsy. Presented at American Academy of Otolaryngology–Head and Neck Surgery. Anaheim, CA, Association for Research in Otolaryngology, 1983. Esslen E. The Acute Facial Palsies. New York: Springer, 1977. Fisch U. Prognostic value of electrical tests in acute facial paralysis. Am J Otol 1984; 5:494-498. Fisch U. Surgery for Bell’s palsy. Arch Otolarygol 1981; 107:1-11. Fisch U, Esslen E. Total intratemproal exposure of the facial nerve. Arch Otolarygol 1972; 95:335-341. Fisch U, Felix H. On the pathogenesis of Bell’s palsy. Acta Otolaryngol 1983; 95(5-6):532-538. 10 Bell's Palsy October 2012 Furuta Y, Fukuda S, Chida E, et al. Reactivation of herpes simplex virus type 1 in patients with Bell’s palsy. J Med Virol 1998; 54:162-166. Freidman RA. The surgical management of Bell’s palsy: a review. Am J Otol 2000; 21:139-144. Gantz BJ, Gmur A, Fisch U. Intraoperative evoked electromyography in Bell’s palsy. Am J Otolaryngol 1982; 3:273-278. Gantz BJ, Rubinstein JT, Gidley P, et al: Surgical management of Bell’s palsy. Laryngoscope 1999; 109:1177-1188. Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:830-836. Hato N, Yamada H, Kohno H, et al. Valacyclovir and prednisolone treatment for Bell’s palsy: a multicenter, randomized, placebo-controlled study. Otol Neurotol 2007; 28(3): 408-413. Ho K. Bell's Palsy: To Treat or Not to Treat. UTMB Department of Otolaryngology Grand Rounds 2007. Accessed 17 Sep 2012 <http://www.utmb.edu/otoref/grnds/Bells-palsy070214/Bells-palsy-070214.mht>. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg 1985; 93:146-147. Katusic SK, Beard CM, Wiederholt WC, et al. Incidence, clinical features, and prognosis in Bell’s palsy, Rochester, Minnesota, 1968-1982. Ann Neurol 1986; 20:622-627. Lockhart P, Daly F, Pitkethly M, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev 2009; 7(4):CD001869. Mancall EL, Brock DG, eds. Gray's Clinical Neuroanatomy: The Anatomic Basis for Clinical Neuroscience, 1st Ed. Philadelphia: Elsevier Saunders 2011. pp 194-198. May M. Total facial nerve in exploration. Laryngoscope 1979; 89:906-917. May M, Klein SR, Taylor FH. Idiopathic (Bell’s) facial palsy: Natural history defies steroid or surgical treatment. Laryngoscope 1985; 95:406-499. May M, Klein SR, Taylor FH. Indications for surgery for Bell’s palsy. Am J Otol 1984; 5:503512. May M, Wette R, Hardin WB Jr, et al. The use of steroids in Bell’s palsy: A prospective controlled study. Laryngoscope 1976; 86:1111-1122. McCormick DP. Herpes-simplex virus as a cause of bell’s palsy. Lancet 1972; 1:937-939. McGovern FH, Hansel JS. Decompression of the facial nerve in experimental Bell’s palsy. Laryngoscope 1955; 71:1090. McKeever P, Proctor B, Proud G. Cranial nerve lesions in Bell’s palsy. Otolaryngol Head Neck Surg 1987; 97:326-327. Murakami S, Mizobuchi M, Nakashiro Y, et al. Bell palsy and herpes simplex virus: identification of viral DNA in endoneural fluid and muscle. Ann Int Med 1996; 124:27-30. Peiterson E. The natural history of bell’s palsy. Am J Otol 1982; 4:107-111. Podvinec M. Facial nerve disorders: Anatomical, histological and clinical aspects. Adv Otorhinolaryngol 1984; 32:124-193. Prescott CA. Idiopathic facial nerve palsy in children and the effect of treatment with steroids. Int J Pediatr Otorhinolaryngol 1987; 13:257-264. Ramsey MJ, DerSimonian R, Holter MR, et al. Corticosteroid treatment for idiopathic facial nerve paralysis: A meta-analysis. Laryngoscope 2000; 110(3 Pt 1):335-341. Salinas RA, Alvarez G, Daly F, Ferreira J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev 2010; 17(3):CD001942. 11 Bell's Palsy October 2012 Salman MS, MacGregor DL. Should children with Bell’s palsy be treated with corticosteroids? A systematic review. J Child Neurol 2001; 16:565-568. Shafshak TS, Essa AY, Bakey FA. The possible contributing factors for the success of steroid therapy in Bell’s palsy: A clinical and electrophysiological study. J Laryngol Otol 1994; 108:940-943. Stankiewicz JA. A review of the published data on steroids and idiopathic facial paralysis. Otolaryngol Head Neck Surg 1987; 97:481-486. Sugita T, Murakami S, Yanagihara N, et al. Facial nerve paralysis induced by herpes simplex virus in mice: an animal model of acute and transient facial paralysis. Ann Otol Rhinol Laryngol 1995; 104:574-581. Williamson IG, Whelan TR. The clinical problem of Bell’s palsy: Is treatment with steroids effective? Br J Gen Pract 1996; 46:743-747. Wolf SM, Wagner JH, Davidson S, et al. Treatment of Bell palsy with prednisone: A prospective, randomized study. Neurology 1978; 28:158-161. 12