Supplementary data for: Improve the activity and selectivity of Fenton
advertisement

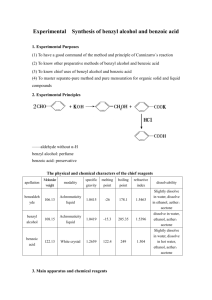
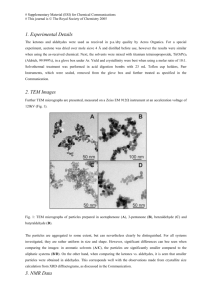
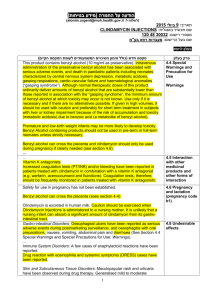
Supplementary data for: Improve the activity and selectivity of Fenton system in the oxidation of alcohols Guoqiang Yang, Qiuxing Lin, Xingbang Hu*, Youting Wu*, Zhibing zhang School of Chemistry and Chemical Engineering, and National Engineering Research Center for Organic Pollution Control and Resource, Nanjing University, Nanjing 210093, P. R. China Table S1. Effect of the amount of catalyst on the oxidation of benzyl alcohol a Entry FeSO4·7H2O NHPI Conv. Sel.b TOF 1 2 3 4 5 6 7 8 (mol%) 0.5 1 2 2.5 2.5 2.5 3.5 5 (%) 3.5 5.4 14.6 36.0 36.7 31.8 34.4 29.1 (/hour) 11.59 9.5 12.9 26.1 27.4 21.9 18.3 10.1 a (mol%) 0.5 1 2 1.25 2.5 5 3.5 5 (%) 99.3(86:14) 99.2(89:11) 99.2(89:11) 98.2(92:8) 99.1(94:6) 95.9(90:10) 99.0(94:6) 97.0(90:10) 20 mmol of benzyl alcohol, 2.2 equiv. of H2O2 (30 wt% in water), catalyst (0.5 mol%-5 mol%), solvent free,25oC,30 min.H2O2 was added with a syringe pump by 0.2 ml/min speed. b Total selectivity to benzaldehyde and benzoic acid(ratio of benzaldehyde/benzoic acid). Table S2. Effect of the temperature on the oxidation of benzyl alcohol a Entry 1 2 3 4 5 a Temp.(oC) 10 25 40 55 70 Conv.(%) 8.6 36.7 50.2 55.0 46.5 Sel.b(%) 99.0(92: 8) 99.1 (94:6) 95.3 (91:9) 93.8(91:9) 93.5(92:8) 20 mmol of benzyl alcohol, 2.2 equiv. of H2O2 (30 wt% in water), solvent free, 30 min. H2O2 was added with a syringe pump by 0.2 ml/min speed. b Total selectivity to benzaldehyde and benzoic acid (ratio of benzaldehyde/benzoic acid). Figure S1. Benzyl alcohol conversion and selectivity with the reaction time. Reaction conditions: 20 mmol of benzyl alcohol , solvent free,25oC,enough H2O2 was continuously added with a syringe pump.&=by 0.3 ml/min speed;&=by 0.2 ml/min speed;&=by 0.15 ml/min speed;◆&◇=by 0.1 ml/min speed. Figure S2. Effect of the iron-based catalysts. Reaction conditions: 20 mmol of benzyl alcohol, 2.2 equiv. of H2O2 (30 wt% in water), Reaction time: 0.5 h. H2O2 was added with a syringe pump by 0.2 ml/min speed.