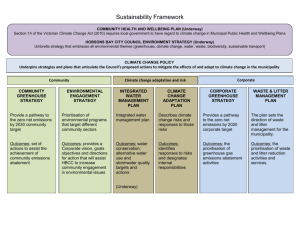
Prioritising Health Services - National Health Committee
advertisement

Prioritising Health Services A background paper for the National Health Committee OCTOBER 2004 The National Advisory Committee on Health and Disability (the National Health Committee or the NHC) is an independent committee appointed by, and reporting directly to, the New Zealand Minister of Health. Members of the National Health Committee Robert Logan (Chair) Geoff Fougere Kevin Hague Riripeti Haretuku Linda Holloway Andrew Moore Althea Page-Carruth Neil Pearce Lynette Stewart Lorna Sullivan (until August 2004) Api Talemaitoga Gwen Tepania-Palmer The National Health Committee would like to thank those who provided comments on earlier drafts of this paper, including Grace Campbell-MacDonald, Judy Kavanagh, and Jackie Cumming. This paper represents the work of the NHC, however, and does not necessarily reflect the views of these commentators. This paper is available on the NHC’s website: http://www.nhc.govt.nz INTRODUCTION.................................................................................................................... 1 EXECUTIVE SUMMARY ..................................................................................................... 2 SECTION ONE: INTERNATIONAL EXPERIENCES ...................................................... 4 INTRODUCTION.................................................................................................................... 4 WHY PRIORITISE?.................................................................................................................... 4 IMPLICIT VS EXPLICIT PRIORITISATION .................................................................................... 5 LEVELS OF PRIORITISATION .................................................................................................... 6 Macro Layer....................................................................................................................... 6 Meso Layer......................................................................................................................... 7 Micro Layer ....................................................................................................................... 7 COMMON INTERNATIONAL THEMES........................................................................... 8 1. THE IMPORTANCE OF VALUES AND PRINCIPLES................................................................... 8 1.1 The Netherlands .......................................................................................................... 8 1.2 Sweden ........................................................................................................................ 9 2. TECHNICAL AND INSTITUTIONAL APPROACHES................................................................. 10 2.1 Technical approaches ............................................................................................... 11 2.2 Critiques of technical approaches ............................................................................ 12 2.3 Institutional approaches ........................................................................................... 14 2.4 “Accountability for Reasonableness”....................................................................... 15 2.5 Shifting approaches: the Norwegian experience ...................................................... 16 3. DEFINING CORE SERVICES AND THE EXCLUSION OF HEALTH SERVICES ............................. 17 3.1 Rationing by exclusion: the Oregon experience ....................................................... 18 3.2 The absence of exclusion .......................................................................................... 19 4. EVIDENCE BASED MEDICINE .............................................................................................. 20 4.1 Clinical Guidelines ................................................................................................... 21 4.2 Technology Assessment ............................................................................................. 22 4.3 EBM in practice: the UK experience ........................................................................ 22 4.4 EBM in practice: the Dutch experience .................................................................... 23 5. INCORPORATING CLINICIANS, PATIENTS AND THE PUBLIC ................................................. 24 5.1 Why involve stakeholders? ........................................................................................ 24 5.2 How to involve stakeholders ..................................................................................... 26 5.3 The NICE approach to stakeholder involvement ...................................................... 28 SUMMARY ............................................................................................................................ 29 SECTION TWO: THE NEW ZEALAND EXPERIENCE................................................ 30 INTRODUCTION.................................................................................................................. 30 THE PURCHASER-PROVIDER SPLIT AND THE ORIGINS OF PRIORITISATION DEBATES ............... 30 OPENING THE DISCUSSION: THE CORE SERVICES COMMITTEE ..................... 31 THE NATIONAL HEALTH COMMITTEE ................................................................................... 32 MACRO LAYER PRIORITISATION ................................................................................ 33 PHARMAC: AN INTERNATIONAL ANOMALY ........................................................................... 33 MICRO LAYER PRIORITISATION AND EVIDENCE BASED MEDICINE ............. 35 CLINICAL GUIDELINES AND THE NEW ZEALAND GUIDELINES GROUP ................................... 35 CLINICAL PRIORITY ACCESS CRITERIA ................................................................................. 36 MESO-LAYER PRIORITISATION ................................................................................... 37 THE REGIONAL HEALTH AUTHORITIES (1997 – 1998) .......................................................... 38 THE HEALTH FUNDING AUTHORITY (1998 – 2000) .............................................................. 38 THE PRESENT PICTURE: DISTRICT HEALTH BOARDS AND DEVOLUTION ................................ 39 ONGOING ISSUES AT THE MESO-LAYER ................................................................................. 42 Staff capacity.................................................................................................................... 42 Political factors................................................................................................................ 42 Macro-layer constraints................................................................................................... 43 Availability of information ............................................................................................... 43 Consistency of approaches .............................................................................................. 44 SUMMARY ............................................................................................................................ 44 APPENDIX: DEVELOPMENT AND ASSESSMENT PROCESSES OF THE NATIONAL INSTITUTE FOR CLINICAL EFFECTIVENESS ..................................... 46 GUIDELINE DEVELOPMENT ................................................................................................... 46 TECHNOLOGY ASSESSMENT .................................................................................................. 47 INTRODUCTION Almost all decisions made in the health sector are, to some extent, prioritisation decisions. We live in an environment of limited resources; not only money, but also time, space, and available staff all constrain the ability of both the system to provide, and people to access, health care services and health promotion programs. Consequently, these resources need to be allocated in some fashion, and unless we allocate them completely randomly – and arguably even then – we are engaging in prioritisation. However, while the role played by politicians, public servants, and administrators in resource allocation might be relatively clear, the full spectrum of those who play a part in prioritisation includes all those with any sort of decision-making power. In particular, health professionals prescribe interventions that require resources and, while the type and features of the intervention may be constrained by other decision-makers, clinicians are therefore the ones who mainly control (in consultation with the people concerned) the demand for resources. Even if a professional ignores all other considerations and prescribes interventions whenever they feel a person will benefit, they are still choosing to allocate resources in a particular way; in this case purely on the basis of effectiveness. Prioritisation is therefore an issue of relevance to all in the health sector, and the question of how we should prioritise underlies almost every policy issue in health and many issues of professional practice. The National Health Committee (NHC) firmly believes that the issue of prioritisation is one that should be discussed as openly and widely as possible both inside the health sector and amongst the general public. This is both an ethical principle – the public has a right to have input into how public resources are allocated – and a practical one. If practitioners and the public do not accept and engage with the need to prioritise, then the prioritisation decisions that are made will likely lead to dissatisfaction and the subversion of processes for doing so. This paper has been produced by the committee to inform its future work programme on prioritisation. It was originally intended as an internal working paper, but the committee believes that this work will also be of interest to others involved, in the widest sense, in the health and disability sector. The paper is structured in two sections, the first of which provides a basic introduction to key concepts in prioritisation, and an overview of themes that have emerged in the international development of explicit prioritisation systems. The second section looks at the development of explicit prioritisation in New Zealand, from the work of the Core Services Committee in the early 1990s through to the current way in which prioritisation is approached at each layer of the New Zealand health sector. 1 EXECUTIVE SUMMARY Internationally, there exists some debate over what precisely constitutes rationing of health interventions. However, the key element all constructions of prioritisation have in common is the notion of scarcity: when need for interventions cannot be met by the supply, some form of priority setting must occur. This can be done either explicitly or implicitly. Traditionally, implicit approaches have dominated health sector decision-making. In these models, decision-makers act without reference to a set of publicly defined criteria. This has the advantage of allowing significant flexibility in the setting of priorities and allocation of resources, but has also been criticised for leading to inefficient and ineffective decisions, as well as being fundamentally at odds with principles of democratic accountability. It may also lead to prioritisation decision-makers at different levels making decisions according to conflicting criteria. Explicit prioritisation, conversely, involves setting priorities through a clear, transparent process and according to publicly defined criteria. Advocates of explicit processes argue that they improve the quality of decision-making and accountability, while opponents claim that they are politically and practically difficult, and cannot account for the variety of contexts in which health interventions can potentially be offered. Whether explicit or implicit, prioritisation decisions can be seen as occurring at three broad layers in publicly funded health systems.1 The first of these is the macro- or political layer, at which decisions are made regarding the determination of the overall health budget and what health interventions will be able to access this public funding. The second is that of the meso- or administrative layer, at which the resources to support the provision of interventions are allocated. Finally, there exists the micro- or clinical layer, at which individual health practitioners, in conjunction with patients, determine who will actually receive access to interventions. Internationally, the development of explicit prioritisation processes has been a difficult and controversial process. However, while the specifics of implementing such systems have varied considerably, there are certain key themes running through the experiences of all jurisdictions. The first of these is the importance of identifying values and principles; there is a common recognition that the concepts that underlie decisions must be clear and publicly discussed. Without this, the results of prioritisation processes can be out-of-step with public opinion and the legitimacy of both the resulting decisions and the process as a whole, can be critically impaired. A second common theme relates to what should be the prime concern in the development of prioritisation processes – a theme that has developed over two phases. In the first of these, “technical” approaches dominated that emphasised the central importance of identifying information and developing fixed rules for its use. These were intended to lead to clear and unambiguous rationales for making decisions. Such approaches have been subject to significant critique, however, leading to a second phase of “institutional” approaches to explicit prioritisation. In these approaches, information in and of itself is seen as of lesser importance than the development of appropriate institutions for using this information. The third major theme relates to the exclusion of specific interventions from the receipt of public funding. While several jurisdictions have attempted to use explicit prioritisation to 2 exclude specific interventions from funding, the State of Oregon in the United States of America is the only one generally considered to have achieved substantial success in this regard. Elsewhere, the strength of professional and practical arguments against such attempts has led to exclusions being implemented to only a very limited extent. The fourth international theme consists of the growth of evidence based medicine (EBM) as an aid to prioritisation decisions. Consisting of two main branches – clinical guidelines and technology assessment – the goal of EBM is to ensure that decisions made in the health system are supported by sound evidence. There remain important questions surrounding EBM, such as the tension between clinical and research settings and the types of knowledge gained from each, and a lack of clarity regarding the actual benefits of implementing evidence based practice. However, there is a clear international trend towards enhancing the use of such techniques – at worst there is no evidence they have a detrimental effect on health outcomes, and their potential positive impact for both individuals and the health system is significant. The final major theme is the need to involve stakeholders in prioritisation decisions. While debate exists over who should be involved, how they should be involved, and the extent to which they should be involved, there is a clear trend toward involving patient groups, health professionals, and other interested groups – including the general public – in decisions over the allocation of health resources. Not only is this trend due to the information stakeholders can provide to higher-level decision-makers, but also on the potential for information to flow in the opposite direction. Involving stakeholders in decisions can enhance awareness of the issues and constraints that surround decision-making, and thus make them more accepting of prioritisation outcomes, or more willing to address the factors that create these constraints. Running through all these major themes is one more common issue: the difficult and controversial nature of prioritisation in general, and establishing explicit prioritisation systems in particular. It is possible to link the previous themes together and thereby talk about a dominant international approach to prioritisation involving the explicit identification of principles, a concentration on processes over technical solutions, placing emphasis on clinical flexibility, promoting evidence based approaches to decision-making, and enhancing the involvement of stakeholders. The translation of this general approach into practice, however, is far from straightforward. 3 SECTION ONE: INTERNATIONAL EXPERIENCES Introduction This section presents a thematic overview of the experiences of countries other than New Zealand in establishing explicit priority setting processes for the publicly funded element of their health sector.i In terms of analysing these experiences, five major themes can be identified: 1. the centrality of discussions over values and principles 2. the contrast between technical and institutional approaches 3. the problematic nature of excluding specific health services from receiving funds 4. the widespread growth of evidence-based medicine 5. the involvement of clinicians, patients and the public in prioritisation. Strung through all these five themes is a further common thread of the tension between the establishment of explicit processes and the place of clinical autonomy and freedom. Before discussing these themes, however, this paper will examine some of the basic questions underlying the development of prioritisation processes: reasons for prioritising, implicit and explicit forms of priority setting, and the different layers at which prioritisation decisions are made. Why prioritise? At heart, discussions about prioritisation are discussions about access to healthcare: who has access to health interventions,ii what health interventions are available to access, and when people are able to access those interventions. The dominant view of prioritisation, often couched in terms drawing on economic market theory, is that priority setting in the health sector is inevitable, as access to healthcare exists in an environment of “scarcity”. Resources that support health interventions are always restricted, relative to the potential demand for them.2 There exists a limited number of health professionals to perform interventions, a limited amount of time in which interventions can be performed, a limited number of areas in which an intervention can take place, and a limited amount of funding to support these interventions. All these factors place limits on the ability of individuals to access health services to some extent – although those limits will be greater or lesser depending on the specific level of resources available. The range of valuable interventions that could potentially be offered, in contrast, is virtually limitless and ever-expanding due to new research, refinement, and technological development. It is worth noting that this paper uses the terms “priority setting”, “prioritisation”, and “rationing” interchangeably. While some have argued that the term “rationing” should be preserved for use only in reference to micro-level prioritisation decisions, this paper follows Ham and Coulter in finding that this terminological distinction adds little.2 Similarly, while the terms “priority setting” and “prioritisation” can be used to refer to different phenomena, both involve the same issues and trends. ii In this paper, the term “health intervention” is used to refer to the entirety of the wide variety of measures and tools used within the health sector to maintain or improve health status. However, while prioritising measures aimed at supporting the lives of people with disabilities involves many of the same issues as other interventions, this paper has been written primarily from the perspective of prioritising health, rather than disability support, services. i 4 This particular construction of rationing is not universally accepted. Light and Hughes, for example, have argued that this definition is so wide that the idea becomes an essentially meaningless concept, and other theorists contend that the term “rationing” should be reserved only for situations in which a person is denied access to interventions that they would otherwise have chosen to receive.2 What all conceptions of prioritisation have in common, however, is the notion that priority setting must occur when there is greater demand for a health intervention than can be met by available resources. Scarcity then, is the key reason for prioritising, and the reason why discussions of prioritisation often go in hand-in-hand with those of health sector funding. Even if prioritisation is essentially an inevitable activity in all modern economic and political systems, the fewer resources available to allocate the more important, yet often more difficult and controversial, such decisions will become. To quote the World Health Organization, “it is difficult to think about priorities at all [in the former Soviet Union] when the national health budget has declined from US$150 to just US¢40 per capita.”3 While prioritisation policy concerns how to allocate available resources, funding policy determines what resources are available to allocate, and thus the two areas are closely linked. Indeed, in some cases discussion of prioritisation processes has actually led to increases in health funding, as it has highlighted the need for further resources if the goals of the health system are to be effectively achieved.4 Implicit vs explicit prioritisation Irrespective of basic assumptions regarding the nature and inevitability of rationing, there is agreement that prioritisation can occur in two ways: implicitly and explicitly. In the first of these, prioritisation decisions are made but the reasoning behind them is not made clear, and access to interventions is rationed without reference to a publicly defined set of criteria. iii Explicit prioritisation in contrast, as the name suggests, involves the establishment of clear, publicly available criteria and processes specifically designed to set priorities. Traditionally, the implicit approach has dominated priority setting in publicly funded health systems. Since the late 1980s, however, there has been increasing international interest in the development of explicit prioritisation processes. Driven by a combination of new health interventions and limited health budgets,4 a desire on the part of some theorists to develop a more “rational” approach to decision-making in health systems,5 and high-profile cases of individuals being denied possible treatment due to a shortage of public resources,6 many jurisdictions have attempted to engage with the question of how to prioritise access to health interventions. As well as New Zealand, the American State of Oregon, Scandinavian countries, the United Kingdom, the Netherlands, the Council of Europe, Israel, and Canada have undertaken major projects on prioritisation, while other jurisdictions such as Australia and Iceland are at least examining which ideas should underlie the prioritisation of access to health interventions. The aims of these jurisdictions have been to consider both how prioritisation decisions should be made and how the quality of these decisions can be improved. However, the development and implementation of explicit approaches to prioritisation has been a difficult and iii Implicit decision-making can involve a shared informal set of criteria collectively used by a group of decisionmakers, or decision-making involving no openly acknowledged criteria whatsoever. In either case, however, the key feature is that the criteria are not made clear to the public at large, nor formally defined in any manner. 5 controversial activity wherever it has been attempted. Not only have specific elements of explicit priority setting processes been criticised, but several theorists have defended implicit prioritisation on the grounds that explicit prioritisation is both politically impractical and incapable of accounting for the diverse contexts in which access to health interventions must be allocated.2 It should also be noted that the development of explicit prioritisation processes has concentrated largely on the funding and allocation of health interventions – rationing expenditure on pharmaceuticals, medical devices, particular procedures and so forth. This focus can be seen as a significant failure, as access to health interventions is dependent on more than the simple availability of funding. Expenditure on physical resources (such as hospital beds) and professional resources (health professionals) are also vital factors that influence access to the health system, but they have been largely absent from discussions of priority setting, and rationing in this area is often left implicit.iv It has been the longstanding position of the National Health Committee, from its origin as the Core Services Committee, that implicit prioritisation is fundamentally less desirable than explicit forms. As the committee argued in 1997: We must stop making decisions about which services are available and who has first call on them behind the scenes and in response to immediate circumstances, rather than in the open and in the wider context … The National Health Committee considers the policy environment of our health and disability support system should be based on conscious and explicit principles.7 Levels of Prioritisation Prioritisation decision-making in the health sector occurs at several different levels. Klein, for example, has identified five different levels of priority setting:1 1. 2. 3. 4. 5. The overall level of funding to be allocated to health services. The distribution of the budget between geographical areas and services. The allocation of resources to particular forms of treatment. The choice of which patients should receive access to treatment. Decisions on how much to spend on individual patients. These levels can be grouped into “macro” (political), “meso” (institutional) and “micro” (individual) layers. In general, levels one and two are macro-layer, level three is institutional, and levels four and five are micro-layer. Macro Layer The most fundamental point at which prioritisation decisions are made is that of central government in its determination of the overall health budget. As governments do not have unlimited resources, and few if any governments are likely to have the required capital to iv To some extent this may reflect the ability of the supply of these resources to be affected by health decisionmakers, as ongoing debates over workforce planning in the health sector suggest. 6 satisfy every possible demand for funding, the government must set the priority of expenditure on the health sector in relation to such areas as education, defence or culture and heritage programs. Of course, the holistic nature of policy means that expenditure on an area nominally outside the health sector (such as housing) may actually have significant health benefits. Prioritisation at this level does not therefore represent prioritisation of “health” per se, so much as prioritisation of funding for those services that receive funding through the health vote.v Below this level is the large-scale allocation of the overall health budget to different elements of the health system. Included in this level is the decision whether or not to exclude particular interventions from receiving public funding, thus restricting their availability to those able to obtain them outside the publicly funded health system. Meso Layer After these two fundamental prioritisation levels comes a third level of prioritisation, where the funding determined at the macro-level is allocated to specific interventions. Prioritisation of capital and staffing investment can also be considered to occur at this level. This level of priority setting usually occurs either within specific institutions such as individual hospitals, or within regional authorities that have responsibility for overseeing the provision of health services within a given district. It is, however, possible for this level to occur at a combination of central government and devolved points if, for example, a government devolves the allocation of most health funding but continues to directly oversee the provision of certain treatments. Micro Layer The final layer of prioritisation is that of clinical decision-making made by individual health practitioners and patients. This involves determining who exactly receives a particular intervention, and for how long. While health professionals are the dominant decision-makers at this point, those requiring health interventions also play a role at this level by agreeing to receive those interventions. The micro-layer may not initially seem like a point where prioritisation occurs. However, as noted earlier, “prioritisation” is essentially about determining access to health interventions, and this micro-layer – although constrained by decisions at higher levels – is the point at which theoretical access to interventions is translated into practice. If the macro- and mesolayers involve prioritising the supply of interventions, micro-layer prioritisation involves decisions relating to their demand. This particular prioritisation layer can be one of the most difficult at which to set priorities, as it involves direct contact with individuals in need of health interventions. Whereas macroand meso-layer prioritisation deal with setting priorities in the abstract, at the micro-layer decisions become far more personal. Health professionals must relate decisions made at higher layers to the specific context in which people present themselves, and given the nearly v It is worth noting that, while most apparent at the macro-layer, this same point can apply to priority-setting at lower points as well. 7 endless variety of factors that can impact on an individual’s need for interventions this can be a highly complex – and often emotionally draining – task. These three layers are best viewed not as neatly-separated stages, but rather portions of a decision-making continuum that involves significant interaction between levels. For example, waiting lists and booking systems, as vehicles whereby health systems and providers organise the provision of health interventions,8 are meso-level prioritisation tools. However, exactly where a given person is placed on a list usually involves a significant level of micro-layer decision-making in, for example, interpreting access guidelines in terms of an individual case. Similarly, the end results of micro-layer prioritisation decisions – most clearly publicity over denial of treatments – can have significant impacts on priority-setting at higher layers. COMMON INTERNATIONAL THEMES 1. The importance of values and principles The most fundamental theme in the development of prioritisation processes is the central role of principles and values in prioritisation. If access to health interventions is to be rationed, then this clearly requires a basis on which such rationing decisions can be made. This does not mean, however, that prioritisation requires active discussion of these principles and values. A key criticism of implicit approaches to priority setting, for example, is precisely that the underlying principles that drive decision-making are hidden.2 Furthermore, identifying values and principles as at the heart of prioritisation immediately begs the question of whose values and principles should be considered in the development of prioritisation processes – an issue that will be returned to in section five of this paper. While there is consensus that values and principles lie at the heart of priority setting, however, the actual principles used to guide prioritisation decisions vary considerably between jurisdictions. Although there are common themes, such as some manner of reflecting the impact of a condition on a person’s quality of life and the cost of providing treatment, the specific values advocated vary widely. The State of Oregon, for example, identified thirteen principles – although it divided these into three main groups – while other jurisdictions have confined themselves to three or four.6 The divergent experiences of Sweden and the Netherlands provide interesting contrasts in terms of the principles used to drive prioritisation. 1.1 The Netherlands Discussions over priority setting first made an appearance in the Netherlands in the 1980s, when the Dutch Health Insurance Council (Zeikenfondsraad) began promoting increased investment in Technology Assessment (discussed below in section four) and subjecting new medical technologies to cost-effectiveness analysis before permitting their use to be publicly funded.9 Prioritisation became a major public issue in 1988, however, with the commencement of a concerted effort to reform expenditure in the Dutch healthcare sector. The primary result of this was the 1991 report by the Committee on Choices in Health Care (the Dunning Committee), which advocated four major strategies for reform:9 8 promoting investment in health technology assessment promoting the use of standardised guidelines and protocols in clinical decisionmaking developing criteria for access to waiting lists and relative priorities within waiting lists establishing a group of core services that should receive public funding, determined through the application of a framework of values and principles. With regard to the last of these strategies, the Dunning Committee proposed four principles that should guide the delineation of core services. These were: is the particular treatment necessary? Is it effective? Is it efficient? And finally, could its payment be left to individual responsibility? Together, these principles were referred to as “Dunning’s Funnel”, due to their hierarchical nature. Priority setting should first involve determining if a service was necessary. If the answer was yes, then decision-makers should proceed to asking whether or not it was effective and so forth. These principles were not simply intended to guide the delineation of core services, however, but were also intended to guide priority setting at all levels of the Dutch healthcare system. Importantly, however, the Dunning Committee also argued that the first principle – that of necessity – should be formulated in different ways depending on the level at which a prioritisation decision was being made. At the macro level this decision should be made from the point of view of the community as a whole, at the meso level from a professional perspective, and at the micro level from the viewpoint of the individual patient.6 The Funnel principles immediately resulted in significant controversy – much of this deriving from the intention that these would be used to exclude services from funding at the macro level. The principle of necessity at this level in particular attracted criticism, as it placed the needs of the community above the needs of the individual patient – the ultimate determinant of whether or not a given treatment would be available through the publicly funded health system would not be the benefit that would accrue to the person receiving it, but rather the benefit that would accrue to society as a whole.6,9 The adverse public reaction to these principles severely hamstrung the Dunning report’s recommendations. 1.2 Sweden In contrast to the Netherlands, Sweden has never attempted to exclude specific services from public funding. The Swedish experience with prioritisation began in 1992 with the appointment of a Parliamentary Priorities Commission (PPC) to advise on the issue. From its inception, this Commission recognised the importance of incorporating widespread input into the establishment of priorities, and made an effort to include viewpoints explicitly representing a range of approaches to healthcare. The Commission also made the ethical, rather than economic, aspect of prioritisation their key concern.10 In other words, the PPC did not see its role as ensuring that the resources of the health system were distributed in an economically efficient manner, but rather as ensuring that the beliefs of the public regarding access to health interventions were reflected in practice. The PPC produced a discussion document to stimulate public input in 1993, and a final report in 1995. In this report, the Commission explicitly rejected the definition of services that should or should not be funded, choosing instead to develop a list of principles that should guide prioritisation decision-making. These principles were:6 9 1. The principle of human dignity: all people are equal in dignity, regardless of personal characteristics and functions in society. 2. The principle of need and solidarity: resources should be committed to the person or activity most in need of them. 3. The principle of cost-efficiency: when choosing between different fields of activity or measures, a reasonable relation between cost and effect, measured in improved health and improved quality of life, should be aimed for. The Commission then used these principles to develop a series of priority categories to aid in priority setting decisions at both the political/administrative (ie; macro and meso) and clinical (ie; micro) layers. Of particular interest in the Swedish case is the relative emphasis placed on the principle of cost-efficiency. Given that prioritisation processes concern the allocation of scarce resources, it is unsurprising that a major element in most prioritisation systems has been ensuring that such resources – primarily financial resources – are spent in an efficient manner. Although the PPC did include this among its principles, it was somewhat unusual in ascribing far less value to it than other jurisdictions. In the first place, the principles promulgated by the Commission were hierarchal, so that principles one and two – both of which focussed primarily on questions of social justice and the qualities of patients rather than services – trumped the economic utility of a particular intervention in determining access to health services. In addition to this, the Commission explicitly rejected the possibility of using principle three to prioritise between treatments for different conditions. The rationale behind this lack of emphasis may stem in large part from cultural attitudes towards healthcare and the health system in Sweden. As Rehnberg points out, belief in ensuring fair and equitable access to health services is a principle that is strongly ingrained in the Swedish health system.11 Consequently, both Swedish policymakers and the public may be more tolerant of a health system that appears economically inefficient if such a system still embodies these principles. Conversely, a system seen to be placing too much emphasis on efficiency at the expense of fairness and equity may be less acceptable than in other jurisdictions. Determining the values and principles that should underlie the rationing of access to health interventions is, however, only the first step in developing explicit prioritisation systems. The real challenge occurs when the attempt is made to translate these principles into practice. 2. Technical and institutional approaches Once principles and values have been defined, the next step in developing an explicit prioritisation process is determining the nature of the process that will be used to make prioritisation decisions. In this respect, there are two main theoretical approaches to developing priority setting systems. It should be emphasised that both of these perspectives still presuppose an explicit process – they both involve clearly articulating the foundation on which prioritisation decisions are made, and, most importantly, a formal recognition that there is a need to ration available health resources. It should also be noted that in practice the distinction between these two schools of thought is often less clear-cut than has been presented here – this discussion emphasises the differences 10 between the two in order to better illustrate their underlying philosophical distinctions. In practice, few of those who adopt a technical view of prioritisation would ignore the need to develop effective institutions, while most institutionalists would admit that there is a need to ensure decisions are based on some form of “good” information. The difference between the two approaches is essentially based on the relative emphasis each gives to the two elements of the prioritisation process. 2.1 Technical approaches Firstly, there exists the technical perspective on making prioritisation decisions. Grounded strongly in rationalist theories of knowledge, policy and decision-making, this model emphasises the importance of having the right information available on which to base such decisions, and the development of an appropriate set of rules to apply when making priority decisions. The aim of this approach is to develop a comparatively objective system of prioritisation: standardised techniques are used to quantify the benefits of providing a particular health service, and from this relative priorities can be determined in a manner that provides a clear, unambiguous rationale for the decisions that have been made. 12 The theoretical ideal of this approach would be a formula that could accurately measure and account for all relevant aspects of health sector decision-making, including the relative importance each should have in such decisions. This, in turn, would minimise the “squeaky wheel” problem – the ability of particular interest groups to illegitimately influence prioritisation decisions – which the technical perspective sees as a particular area of concern. Successful technical approaches depend on three main requirements:12 Clarity about objectives. Technical approaches are fundamentally “problemsolving” approaches. As a result, they require precise identification of what is to be achieved through the process, and relevant parameters and context. So a prioritisation system should, for example, be as specific as possible about the purpose(s) of the health system. Information about costs and outcomes. For technical approaches to work they require information on how effective interventions are, and the costs of providing these interventions. This is required so that the relative value of interventions can be determined. Performance measurement. Finally, it is vital for there to exist an effective measure of determining how well the prioritisation process is achieving its goals. This involves determining both how the objectives should be measured, and the specific processes for actually measuring them. The defining feature of technical approaches is the central role they give to the second of the above requirements: information, and the development of appropriate tools to obtain it. The clearest examples of such tools are those drawing on techniques used in health economics, with other main tools being based on needs assessment.13 For example, Cost-Utility Analysis (CUA), probably the most well-developed technical prioritisation tool, is an economic methodology in which the value of a given health intervention is quantified in terms of the number of Quality-Adjusted Life Years (QALYs)vi gained as a result of this intervention, and vi QALYs (and other comparable measurements) attempt to measure the benefits of health interventions by determining an individual’s estimated life expectancy and adjusting it based on the negative impacts of a condition they suffer. So, for example, a person who would normally be expected to live for 50 more years 11 this number compared to the expense of delivering the intervention. In a CUA-based approach the greater the ratio of QALYs to the cost of delivery, the higher the priority for providing access to an intervention. Essentially, technical approaches are primarily concerned with the inputs to decision-making processes – identifying the information that should be used in decision-making, developing a tool for accurately capturing this information, and then ensuring that this tool is used appropriately. In practice, of course, tools will operate within a political environment that makes a fully technical approach at least very difficult to implement.14 However, advocates of technical approaches argue that the key driver of decision-making should be the results of this technical analysis, and a “good” set of prioritisation decisions is one that matches up as closely as possible with that argued for by this measurement. 2.2 Critiques of technical approaches Although technical approaches are attractive to prioritisation decision-makers, they have been the subject of many critiques grounded in both theoretical and more practical methodological issues. While many of these critiques relate to particular aspects of specific technical approaches, a recurrent theme is a questioning of the underlying assumption of technical approaches: that it is possible to develop a tool that can accurately capture and quantify the factors involved in making prioritisation decisions. The first of these critiques centres on the position of values and principles in setting priorities. As noted earlier, all prioritisation decisions, whether explicit or implicit, will be based on certain core beliefs as to the relative value of particular clinical outcomes. For example, prioritising intensive care for those with life-threatening conditions over palliative care for the terminally ill involves an assumption that increasing the length of life is more important than increasing the quality of life.10 Beyond this, prioritisation processes must also include consideration of wider ethical and moral considerations – for example, what role notions of social equity and human rights should play in determining health funding. This is one of the reasons why explicitly identifying the values and principles underlying prioritisation should be the first step in developing an explicit method of priority-setting. For many, however, technical analytical tools – particularly those based on QALYs or its cousins the Disability Adjusted Life-Year (DALY) and Healthy Year Equivalent (HYE) – are ill-suited for capturing these values. Questions of whether age should play a role in determining access to healthcare, whether lives lived with an impairment are truly of less “value”, or if more priority should be given to those less able to afford healthcare, represent fundamental ethical and moral issues that are very hard to analyse within any technical model. In Klein’s words, “the debate about priorities will never be finally resolved. Nor should we expect any final resolution. As medical technology, the economic and from the point of measurement would have a QALY-based life expectancy of only 25 years if they experienced a condition deemed to reduce their quality of life by one half (0.5 x 50 years = 25 QALYs). Measuring the benefit of an intervention in terms of QALYs involves determining how many additional QALYs a person can expect to receive from that intervention. In the previous example, an intervention that raised this person’s quality of life value from 0.5 to 0.75 would have a QALY value of 12.5 (0.75 x 50 years = 37.5 QALYs, – 25 QALYs = 12.5 QALYs). Conversely, one that raised the quality of life value to only 0.6 but increased overall life expectancy to 60 years would have a QALY value of 11 (0.6 x 60 years = 36 QALYs, – 25 QALYs = 11 QALYs). It should be noted that this example is a simplified abstraction and debate continues over precisely how to determine QALYs. 12 demographic environment, and social attitudes change, so almost certainly will our priorities.”1 It is difficult for technical approaches, which by their very nature require the definition of relatively fixed understandings of values and principles, to reflect this fluidity. Consequently, over-reliance on technical tools in making prioritisation decisions can lead to results that are out of step with wider public opinion. Oregon’s first attempt at prioritisation, for example, followed a strongly technocratic model and resulted in such anomalous results as tooth-capping being ranked as of greater value, in terms of offering greater benefit relative to cost, than appendectomies.4 Furthermore, there exists a significant current of opinion that the attempt to develop a “rational”/technical approach to prioritisation is an inherently doomed project. There are two forms of this particular critique. The first of these relates to deep and fundamental theoretical debates about the nature of both knowledge and policy-making, and discussion of these issues are beyond the scope of this paper. A more moderate form, however, argues that technical approaches require information that in practice cannot be gathered, and in many cases is inherently subjective and contestable.1,5 For example, Holm has identified an example of this inherently uncertain and subjective nature of medical practice in the concept of severity – a characteristic most would agree should be an element of any priority setting decision-making: The severity of a disease is open to different interpretations. Whether a disease is lethal or likely to lead to permanent handicap or disability is an aspect of its severity, but severity also includes current state of health (for example, whether there is severe pain or current disability), urgency of treatment, and also the possibility of treating the disease. The severity of the disease thus turns out to be a multifaceted concept…15 As this quote illustrates, what may initially seem to be straightforward qualities often, once they are “unpacked”, turn out to be complex and not amenable to a single definition. While one can attempt to develop general guidelines as to what constitutes concepts such as “severity”, these multiple aspects make definitions and measurements of such variable from case to case and sometimes practitioner to practitioner. From the perspective of this critique, one of the fundamental requirements for a technical approach to work – that of relatively standardised, objective information – often does not exist in the health sector. This is particularly the case when one considers the distinction between “vertical” and “horizontal” priority-setting. The first of these relates to setting priorities between interventions for the same condition or similar methods of attaining one goal – different treatments for chronic heart disease, for example, or two different anti-smoking programmes. In these cases, the interventions being compared are similar enough in approach and desired outcomes that measuring their comparative value may be relatively straightforward and uncontroversial. Horizontal prioritisation, on the other hand, refers to setting priorities for interventions between conditions or with radically different aims – such as prioritising treatments for bowel cancer against hip replacements, or increased expenditure on disability support services against resourcing an anti-obesity campaign. The divergent outcomes here make it difficult to compare the benefit of providing an intervention, as there is no pre-existing “common currency” through which outcomes can be compared.6 13 This is, in fact, the goal driving attempts to construct QALY-style systems: the development of a single metric for determining value between interventions. However, these systems encounter the same stumbling blocks described earlier. Arnesen and Kapiriri, for example, have demonstrated the sensitivity of DALY measurements to value choices that may not be immediately obvious – and the consequent importance of this for health policy: “The creators of DALYs may be well aware of how to disentangle DALYs and of the effect of the value choices but the problem is that the users of the information are probably not.”16 Those who advocate technical approaches to prioritisation certainly acknowledge these issues. However, they also believe that these are surmountable problems. For some, resolving them is essentially a matter of refining the method of information collection and analysis until it accounts for all these intricacies. With regard to the Oregon anomaly noted above, for example, it can be argued that this was simply the result of an imbalance in the quality of the data available for each intervention. Others claim that, while the above critiques are partially justified, focussing on information will still improve decision-making; other problems may remain, but the better information that is available the less of an issue they become.17 For many, however, the issues raised above make concentrating primarily on improving information a case of missing the point at best, and actually dangerous at worst. 2.3 Institutional approaches Scepticism at the ability of technical approaches to “solve” the prioritisation issue does not, however, necessarily entail advocating a retreat from explicit prioritisation as a whole. Recent years have seen the emergence of the institutional or “pluralistic bargaining” approach to priority setting; what Ham and Coulter refer to as the “new synthesis” between implicit rationing and technical approaches.4 The central difference between institutionalism and the technical approach lies in their conception of what the establishment of priority setting processes should be concerned with. As noted earlier, technical approaches to priority setting are based on improving information so that there appears a clear, single answer to the question of “how should we prioritise?” The type of information required and method of obtaining it may vary depending on associated issues (for example, how we define the purpose of the health system), but in essence such approaches posit the existence of a problem – “we are not prioritising effectively” – and advocate a solution – “use tool X to measure value.” In this context, the value of a given intervention is seen as relatively fixed, and something that can be determined through the use of a sophisticated enough technical tool: “the use of QALYs, and other forms of utility assessment, is dependent on … reflecting “reality”, or at least modelling “reality” in an acceptable fashion.”18 For advocates of an institutional approach to prioritisation, however, priority setting is not a puzzle requiring a solution, but rather an ongoing process without a single correct answer.19 From this point of view, rationing access to health interventions is fundamentally a political and social exercise that involves bargaining between those with different interests. This leads to a fundamentally different locus for prioritisation debates. Technical approaches concentrate on identifying appropriate types of information and methods for measuring it. For institutionalists, however, focusing primarily on information fails to account for the context in which prioritisation decisions are made. The clinical setting is too uncertain, and constructions of value are too divergent, for a single method to accurately capture all relevant 14 aspects of health sector decision-making and satisfy all parties. All such methods will be open to challenge and critique, and attempting to impose a single, definitive prioritisation formula will simply lead to anger and frustration amongst all parties. Consequently, instead of positioning information as the centrepiece of priority-setting, institutionalists focus mainly on what is done with information once it is collected. It is the method whereby different values and types of information are mediated that is the prime concern of institutional approaches – their defining feature is a concentration on the process whereby priority-setting decisions are made, rather than the tools used to make them. Of course, technical approaches do not neglect the question of process. However, in such paradigms the process is essentially a means to an end – the aim is to produce a process that supports using information to provide the “right” answer. Institutional approaches, conversely, focus on the process as an end in itself, and accept that the “right” answer in a given situation may not always be the same as that indicated by technical analysis. Correct prioritisation decisions are not those that accord with maximising particular measurements, but rather those that are reached through a process that the majority of stakeholders, even if they disagree with the outcome of that process, can agree has been an acceptable one. Whether the process meets particular ideas of rational decision-making, or the resulting decisions line up with the results of technical analysis, is less important than the fact that all those with an interest in the setting of priorities accept the process and feel that it provides for adequate consideration of their views. 2.4 “Accountability for Reasonableness” Moving away from purely technical approaches does, however, beg the question of how we can determine that an institutional approach is “working”.12 Technical approaches provide a clear basis for this, in terms of how closely decisions match up with the results of analysis. Institutional approaches, however, do not have a similar comparator “built-in” to their design, and instead require one to be developed. In this respect, the “Accountability for Reasonableness” approach advocated by Daniels and Sabin has been influential in theorising how explicit prioritisation processes should operate. Although initially developed to apply to managed care plans in the United States, the general concepts of this approach are applicable to decision-making in all health systems – and arguably to policy-making decisions in general. The notion of “Accountability for Reasonableness” is: the idea that the rationales for [health care] … decisions should not only be publicly available, but should also be those that “fair-minded” people can agree are relevant to pursuing appropriate patient care under necessary resource constraints.20 “Fair-minded people” in this context are those who are committed to making a process work – people who accept that there will be constraints on access to health interventions, and are willing to participate in discussions that will require mediating and accounting for the interests of multiple stakeholders. Daniels and Sabin argue that for this form of accountability to be apparent, that is, for a given decision-making process to be regarded as fair, four conditions should be met:20 15 Publicity Condition: the rationale for decisions (and, indeed, the decisions themselves) must be made freely available to the public. This satisfies the basic ethical principle that people are entitled to know why decisions that affect them have been made. Relevance Condition: the evidence, principles and ideas that underlie the rationale for decisions must be acceptable to all “fair-minded” parties. Appeals Condition: there should exist a system whereby decisions can be challenged and/or revised in the light of further evidence. Enforcement Condition: there exists some system of ensuring that the above three conditions are met. The approach of Daniels and Sabin represents a meta-approach to the question of how prioritisation decisions should be made: a process for thinking about processes. The extent to which these criteria are fulfilled in practice varies considerably between both individual criteria and particular jurisdictions.21 However, this in itself demonstrates that the idea of accountability for reasonableness provides a useful way of evaluating the operation of institutional approaches to priority setting. 2.5 Shifting approaches: the Norwegian experience Initially, technical approaches dominated attempts to develop prioritisation systems. The best-known example of such a technically-dominated system is that developed, and still used, in Oregon.vii Although initial reliance solely on theoretical models led to unworkable results, such as the previously cited example of teeth-capping being ranked as more valuable than appendectomies, refinements to the tool involving the incorporation of public input and ethico-moral principles resulted in a more acceptable priority list – albeit one which has consistently expanded to cover more and more interventions. In more recent years, however, the balance has shifted and internationally institutional approaches now dominate prioritisation systems. Holm, for, example, has identified two phases of priority setting in Scandinavia: an initial period dominated by technical approaches, followed by the identification of major problems with resulting processes and a consequent shift towards institutional perspectives.15 The Norwegian experience provides a good example of Holm’s shift between phases. Norway was the first country to attempt to implement an explicit priority setting system in 1987, with the report of the Lønning Commission. The system implemented as a result of the Commission’s report, unlike Oregon’s, did not create a prioritised list of individual conditions and treatments, but rather created a series of five hierarchical groups based on severity, with clinicians assigning their patients to these groups based on the consequences of not undertaking treatment.22,viii In practice, however, this system proved less than satisfactory, with its vulnerability to pressure from particular patient groups and the actions of individual clinicians particularly important failings.15 As a result of these failings, a new Commission (referred to as “Lønning II”) was appointed in 1996 to recommend changes to the prioritisation system. The report of the Commission vii The approach adopted by Oregon is discussed below in section three. This represents an example of a technical approach based on needs assessment, as opposed to Oregon’s emphasis of economic models. viii 16 proposed a new three-step approach to prioritisation with stronger links between the political and clinical levels of priority setting.22 The Lønning II process begins with specialty-specific working groups, who define the concepts of severity, efficiency and benefit of intervention within this clinical area and then use these to prioritise the conditions found within the specialty. This advice is then passed up to political and administrative decision-makers to use in funding decisions, and also informs the development of clinical guidelines for individual practitioners. The focus of the Lønning II model is not on “correctly” prioritising treatments, but rather on producing prioritisation decisions that enjoy legitimacy and support from the public, patients and health professionals.22 The requirement that the working groups define severity, efficiency and benefit is intended to make transparent the underlying bases on which prioritisation decisions are made, while the involvement of practitioners as an integral element of the process enhances the likelihood that health professionals will not attempt to subvert prioritisation decisions. However, although Norway’s shift in approach is a good example of how attitudes to prioritisation have changed, from the accountability for reasonableness perspective there still exist important flaws in the process. In particular both the publicity and relevance conditions referenced above are met only occasionally,22 and while a well-developed process has been established to appeal prioritisation decisions, Norway follows most other jurisdictions in having no form of enforcement of the type envisaged by Daniels and Sabin.21 3. Defining core services and the exclusion of health services As noted in this paper’s introduction, a central driver of recent increased interest in prioritisation has been a dramatic increase in the number and availability of new health interventions. As a result of this, and consequent pressures on health budgets, one of the key issues facing decision-makers has been the definition of core services. This issue has two linked elements: firstly, the question of “core services” proper – identifying interventions (or groups of interventions) that macro-layer decision-makers should explicitly commit to ensuring will always be available to the public through the publicly-supported health system.ix Secondly, there exists the question of exclusions – should there be interventions, and if so which ones, that macro-layer decision-makers deem should not be supported by public resources or receive such support only in limited circumstances? At heart, however, the core services and exclusion debates are not simply about excluding or including interventions – rather they concern issues of power and authority: where exactly the cut-off point should exist between macro- and meso-layer decision-makers in prioritising the allocation of funding, or even whether all interventions should theoretically be available and micro-layer decisions be the only determinant of whether or not a given person receives an intervention. At one extreme the macro-layer simply allocates an overall quantum of funding to meso-layer decision-makers, who then have complete freedom to spend this in whatever manner they wish. At the other, the macro-layer tightly defines exactly how its funding should be allocated, and the role of the meso-layer becomes essentially one purely of implementation rather than decision-making. Within these two extremes, a variety of ix Whether through a directly publicly-operated health sector, or through providing support for accessing interventions through the private health sector. 17 possible arrangements exist – longer lists of core or excluded services provide greater constraints on meso-layer decision-makers, while shorter lists diminish the power of macrolayer decision-makers. 3.1 Rationing by exclusion: the Oregon experience Of the two “ends” of the core services debate, attention has largely focussed on the issue of excluding treatments. Consequently, one of the explicit prioritisation processes to attract the greatest international attention has been that of Oregon in the United States of America (USA). Although Norway was the first jurisdiction to attempt to explicitly set priorities to guide healthcare funding,23 Oregon attracted international attention not only for its attempt to develop priorities for the allocation of funding but also because the process was developed with the explicit intention of excluding some interventions from public support. The development of explicit rationing processes in Oregon began at the end of the 1980s. Although individual States in the USA had previously established some general health service priorities to cover possible reductions in state budgets, Oregon was the first to attempt to formulate priorities for specific conditions and treatments.24 Oregon’s decision to do so was the result of widespread public discontent at the State’s decision to withdraw Medicaid coverage for certain organ transplants in favour of extended prenatal care services – a policy which led to the death of a child with leukaemia.x,25 Consequently, in 1989 the State legislature established a Health Services Commission that proposed Medicaid coverage be extended to cover all people with incomes falling under the Federal Poverty Line. In order to contain the costs of this expansion, however, it was proposed that access to primary and acute health care services – in other words, the specific conditions for which Medicaid would subsidise appropriate interventions – be restricted.xi For all Medicaid residents subject to the program, the benefit package would be determined by a prioritized list of health services in which health conditions and their treatments are listed by importance from highest to lowest. The State legislature would then determine its budget for the program, and a line would be drawn where projected program costs equal the budgeted amount. All conditions and treatments at and above the line would then be covered; conditions and treatments below the line would not be covered.24 The cut-off point for funding of a given treatment-condition pair in this system could rise or descend biennially with the overall level of Medicaid funding in the State budget, while the rankings on the prioritised list itself would be reviewed every three years. The process of establishing the prioritised list itself was controversial and problematic – as noted earlier, the initial list of services resulted in some anomalous rankings. In general, however, the Oregon list has been accepted as a qualified success. While important flaws x Medicaid is a national insurance program that provides funds intended to ensure that specific groups of lowincome individuals and families are able to access necessary medical care within the USA’s private healthcare system. Although established by federal statute and part-funded by the federal government, individual states are responsible for the actual administration and implementation of Medicaid. This includes: establishing specific eligibility criteria for Medicaid support; determining the type, amount, duration and scope of health services Medicaid will fund; and the level of funding provided by the program. xi Long-term healthcare services were to be excluded from this restriction. 18 remain, the Oregon plan has been responsible for significant increases in the number of people with access to Medicaid services.25 3.2 The absence of exclusion Although the Oregon experience has attracted significant attention and is generally considered a success, the actual influence of this approach to prioritisation has been relatively minor. In the wake of Oregon’s moves, several other jurisdictions attempted to develop a core/non-core distinction. However, such proposals have generally either failed to be implemented or been introduced at only a minor level. The Netherlands, for example, has implemented a very limited form of exclusion-based prioritisation in which a few specific treatments (such as in vitro fertilisation) are not totally excluded from receiving public funding, but have limits placed on the circumstances in which they are so funded.26 This general dearth of exclusion-based approaches to prioritisation is based on a variety of factors. Firstly, excluding services from coverage can face significant resistance from both health professionals and the wider public. One of the advantages enjoyed by decision-makers in Oregon was that the prioritisation process was framed within the context of increasing access to the State’s Medicaid program. Consequently, reductions in medical coverage were balanced by correlating increases in population coverage. This situation is significantly different, however, in jurisdictions with an extensive public healthcare system. Making tradeoffs between the comprehensiveness of service provision and the extent of population coverage is more difficult in [systems other than Oregon’s] … because in these systems there is already a commitment to universal coverage. Setting priorities by excluding services therefore poses political problems because no compensating benefits are on offer.6 Thus, while there may be strong arguments in favour of decisions to withdraw funding for particular health interventions, the loss of previous benefits without any corresponding gain may be unpopular amongst the public. This is less of an issue with regard to the introduction of new interventions. However, such interventions are often both available overseas and in other jurisdictions, and this can lead to significant public discontent regarding decisions not to fund particular interventions. The undoubted position of political pressure (or, to be charitable, the responsiveness of administrations to the wishes of the public) as one factor in the reluctance of governments to exclude specific treatments from public funding has led to criticisms that this is a case of central government avoiding responsibility for making difficult decisions. From this perspective, focusing on processes that should guide prioritisation decisions made at the meso- and micro-levels is seen as an easy out whereby governments can avoid making politically unpopular decisions themselves, and instead require bodies such as local health authorities or health practitioners to bear the brunt of discontent with prioritisation decisions. The renewed … focus on the micro level of rationing can be seen as an example of blame avoidance as decision makers respond to the political costs of being explicit about priorities… One of the effects of such an approach is to direct attention away from relatively visible and accountable decisionmakers to a much more diffuse group.27 19 To characterise lack of political will as the sole motivation for the unwillingness of jurisdictions to take an exclusion-based approach to prioritisation is, however, both unfair and inaccurate. Whether or not political palatability has played a role, there exist other important reasons why governments may be reluctant to exclude treatments from public funding – primarily the restrictive nature of prioritised lists of interventions. By their very nature, such lists are based on a constant measurement of value – they assume that a given condition will always have a given equal “negative” value and, correspondingly, that treatment of this condition will therefore have a consistent level of health benefit. However this value is determined, prioritised lists are based on the assumption that it is both possible and desirable to denote service B as always delivering more benefits than service C, and less value than service A. Even more sophisticated versions of this approach, which attempt to allow for the characteristics of specific cases, still rely on an assumption that it is possible to define the value of an intervention to members of a particular group. In practice, however, this assumption is difficult to support. People experience need for health interventions within an almost endless diversity of contexts and characteristics that can all affect each other, and the benefit to an individual (and others, such as family members) from a particular intervention can therefore vary immensely. As the UK Department of Health argued in 1995: To attempt to draw up national lists of treatments which will and will not be provided would be an exercise fraught with danger. No one list could ever hope to accommodate the range and complexity of the different cases which individual clinicians face all the time.28 In essence, defining core services rests on the construction of a typical context in which decisions regarding health interventions can be made. This ignores the fact, however, that specific contexts – and therefore specific benefits – can vary greatly from this norm. While there exist interventions that may commonly provide only low benefits, there is always the possibility of a case where undertaking the intervention will result in significant gains. Preventing an intervention from being accessed through the public health system removes the system’s ability to account for such situations. 4. Evidence based medicine Exclusion approaches are an example of decision-making processes at the macro-layer. With their general failure, attention has increasingly moved to prioritisation at the meso- and micro-layers. Central to this has been the recent growth of evidence based medicine (EBM). The founding principle of the evidence based medicine movement is the claim that much of, or even most, existing clinical practice is not supported by sound scientific evidence and hence does not constitute the most effective use of limited healthcare resources.6 As a result, advocates of EBM argue for increased research into the efficacy of both new and existing treatments, improved information flows between researchers, clinicians and policy-makers, and a willingness to discard established practices when evidence does not support their use. Although EBM may have begun purely as an attempt to improve clinical practice, its value in terms of prioritisation is obvious. If the movement’s position is accurate, then pursuing 20 evidence-based medicine not only has the potential to improve the clinical care of patients, but may also ensure that resources are not used when their value is questionable. Importantly, however, an increase in evidence based approaches does not inevitably lead to reductions in costs. A corollary of willingness to discard established practices is the willingness to maintain existing expenditure and provide new interventions when evidence supports this. Several important critiques have been made of the evidence based movement. For example, the particular form of “evidence” advocated as the gold standard by the evidence-based movement – usually double-blind randomised clinical trials – is more suitable for assessing some interventions than others.13 This has particularly important implications for horizontal priority setting. The most influential opposition has come from clinicians who argue that the health environment is an inherently uncertain one that bears little resemblance to that of a clinical trial, and for which the standardised practices that are the goal of EBM are unsuitable and no replacement for individual clinical judgement – a viewpoint that has resulted in health practitioners continuing to rely on experience and precedent even when it conflicts with dominant scientific opinion.6,29 Despite these critiques, however, the evidence-based approach has become highly influential in thinking about health practice and policy. In terms of prioritisation, EBM has progressed in two linked streams: the development of clinical guidelines, and promotion of technology assessment. 4.1 Clinical Guidelines If excluding interventions is a “top-down” approach to prioritisation in which the potential to offer an intervention is eliminated at the macro-layer, clinical guidelines constitute the polar opposite. The guidelines approach instead focuses on the opposite end of the health system and the micro-layer of priority setting in which the decision is made to offer an intervention. Rather than determining the parameters within which practitioners make prioritisation decisions (ie; the availability or otherwise of specific health interventions), clinical guidelines attempt to influence the actual making of those decisions. They do this by establishing “best practice” advice on when, how long, and in conjunction with what other interventions a given health intervention should be prescribed by a health professional. This is seen to both maximise the potential efficacy of an intervention, and ensure that interventions are not offered when they will be ineffective or unnecessary. One of the key advantages in the development of clinical guidelines is their flexibility. As examples of best practice, their role is to provide general advice on the use of health interventions and promote a coherent approach to treatment between health professionals. At the same time, however, their status as guidelines rather than rigid protocols preserves the autonomy necessary for health practitioners to exercise their own clinical experience and judgement in addressing the specific needs of individual patients. While clinical guidelines are generally seen in a positive light, however, their actual impact remains unclear. On an implementation level, many health professionals are cautious of using guidelines – mainly due to the general objections raised against EBM noted earlier – and internationally their use by practitioners remains low.30 Furthermore, and somewhat ironically, evidence as to the actual impact of guidelines on health outcomes is unclear. 21 While there is no evidence that clinical guidelines worsen health outcomes, the benefits, if any, stemming from their use appear to be relatively small and highly variable between cases.30 4.2 Technology Assessment In many ways, technology assessment (TA)xii forms the core of the evidence-based medicine movement. The purpose of technology assessment is to develop a measure of the effectiveness of a given medical procedure, appliance, pharmaceutical, and, in at least some formulations, new systems and structures as well – including evaluation of the technology’s medical efficacy, questions of safety, and possibly the ethical, social and financial implications of introducing it.31 Technology assessment is of significant value in prioritisation. Firstly, for those who advocate technical approaches to priority setting the information provided by health intervention assessment is vital in ensuring that such approaches are workable. Technical approaches require detailed and specific information, and health intervention assessment is a key vehicle for obtaining this information. Furthermore, unlike clinical guidelines, which are focussed on the micro-level of prioritisation, TA is usable at all three prioritisation layers. At the macro and meso layers, TA provides information to guide overall resource allocation.xiii At the micro-level, technology assessment both provides advice to individual practitioners and supports the development of clinical guidelines. 4.3 EBM in practice: the UK experience Internationally, one of the most well-developed examples of EBM-based priority setting is that of the National Institute for Clinical Excellence (NICE) in the United Kingdom. The Institute undertakes both technology assessment (technology appraisals) and the formulation of guidance for the management of specific health conditions (clinical guidelines) for the National Health Service (NHS) in England and Wales.xiv Organised technology assessment in the UK began in the early 1990s, with the development of a national health technology assessment program in 1993 and the creation of the NHS Centre for Reviews and Dissemination (CRD) in 1994, both of which were intended to improve the availability of information about the effectiveness of health technologies for clinicians and other decision-makers. However, the effectiveness of these two institutions is Although “technology assessment” constitutes standard terminology, the term “health intervention assessment” is also used to refer to the same phenomenon. Using this second term has the advantage of avoiding the connotations of physical devices possessed by the word “technology” and thus arguably more accurately reflects the range of procedures, devices and pharmaceuticals that can be assessed under this overall rubric. This paper uses the terms interchangeably (though only the abbreviation TA). xiii The introduction of some new technologies, such as pharmaceuticals, is one of the few areas in which exclusion-based approaches to prioritisation have been relatively effective. Technology assessment can also be used in financial planning, by predicting how often situations where a given intervention is useful are likely to arise and thus likely demand for the technology. xiv For the NHS in Scotland two separate bodies, the Health Technology Board and the Scottish Intercollegiate Guidelines Network, undertake technology assessment and the development of clinical guidelines respectively. Both do, however, work closely with NICE in developing their advice. The Institute also now provides advice on the safety of interventional procedures, and this advice covers the NHS in England, Wales and Scotland. xii 22 questionable, and the assessment of health technologies was still largely undertaken in an ad hoc and disorganised manner well into the late 1990s.32 As a result of dissatisfaction with these existing arrangements for health technology assessment and several high-profile public debates over the use of particular pharmaceuticals,33 1999 saw the establishment of NICE to promote a coherent approach to promoting the effective use of health interventions. The Institute also acts as a vehicle for involving the public and stakeholders in the formulation of guidelines and technology assessment, so that the advice it provides includes an appreciation of factors beyond pure clinical effectiveness. NICE does not itself develop guidelines or undertake assessments – instead, it operates as a co-ordinating and facilitating body for evidence based medicine. Once it has been determined that a particular intervention should be examined or guideline formulated, it brings together groups of practitioners and researchers to develop both the scope of the activity and produce the guidelines or assessment. At the same time, NICE acts as a guarantor for the process by setting out a specific oversight arrangement involving both the Institute itself and groups of stakeholders. More detailed discussion of the Institute’s processes for guideline development and technology assessment can be found in the attached appendix. However, while NICE’s processes may be well-developed, the exact impact of its guidelines and assessments remains unclear. There exists some evidence that specific recommendations are being routinely ignored, while conversely it has been claimed that the money saved by recommendations for decreased use of some interventions has been overcompensated for by other recommendations for increased use of other treatments by approximately 200 million pounds.33 It has been argued that one reason for this is the Institute’s focus on the examination of new treatments and relative neglect of older, existing technologies. The NHS cannot afford NICE generosity, even with increased funding, because of the resource demands of other access and national service framework targets, many of which have yet to evaluated by NICE.34 4.4 EBM in practice: the Dutch experience The difficulty NICE has experienced in having its advice adopted by practitioners and decision-makers mirrors overall international experiences. The Netherlands, another jurisdiction where extensive use of EBM has been promoted as a means of addressing priority setting issues, has also experienced significant problems in translating this into practice. As noted in section one of this paper, discussions over priority setting in the Netherlands began in the 1980s with the Zeikenfondsraad’s recommendations that all new medical technologies be analysed to determine their cost-effectiveness. The Dunning Committee’s 1991 report re-emphasised the importance of TA, and such assessment continued to be enthusiastically promoted as the rest of the committee’s recommendations fell by the wayside.9 At the same time, the Netherlands has followed other countries in promoting the use of clinical guidelines. In contrast to the rhetoric, however, the practical impact of technology assessment on the Dutch health system has been patchy. 23 At a structural level, both health intervention assessment and the development of clinical guidelines occur haphazardly, with a large range of central and local bodies undertaking technology assessment and guideline development in a haphazard and uncoordinated manner. For example, both the Dutch Institute for Healthcare Improvement (CBO) and the Dutch College of General Practitioners (NHG) have produced guidelines covering treatment of the same conditions, and there is little linkage between the two branches of EBM.9 This situation is replicated in terms of translating these EBM measures into practice. Research has also found that Dutch health professionals pay little attention to guidelines and that many practitioners are unaware of their content, and that there is little evidence of technology assessment having significant influence on policy-making.9 5. Incorporating clinicians, patients and the public The final key theme in international experiences of prioritisation is the need to involve stakeholders in the process of setting priorities. Indeed, one of the key reasons that jurisdictions have shifted away from technical approaches is a perception that technical approaches fail to engage with stakeholders and privilege the perspectives of a small group of decision-makers. Before discussing this, it should be noted that health service administrators and technical analysts constitute a group of stakeholders in their own right – administrators and analysts have their own particular interests and positions regarding prioritisation, 35 and it is important to recognise this fact. However, this group is, by their very nature, involved in decision-making processes, and therefore “stakeholders” in the context of this paper will be used to refer to decision-makers other than administrators. 5.1 Why involve stakeholders? In the context of prioritisation, stakeholders can be divided into three core groups: practitioners, patients and the general public. There are three major arguments for ensuring that stakeholders play an important part in prioritisation processes. The first of these is an ethical one: in situations where the public funds a health care system, the public has a right to some input into how those resources are allocated.19 In the context of prioritisation, this is particularly important given the previously noted importance of values and principles in the setting of priorities, and the consequent need to identify these. These cannot be simply assumed, as the actual preferences of individuals (in terms of the value they ascribe to particular health states) can differ markedly from those predicted by theoretical models and, critically, from those values held by policymakers and technical experts.19,35 Furthermore, it has been argued that increasing the involvement of the public generally enhances accountability by requiring bodies responsible for priority setting to be more explicit about the reasons for the decisions they make.36 A second rationale for increasing stakeholder involvement in decision-making processes stems from an appreciation for the varying perspectives, understandings, and forms of knowledge that different stakeholders bring to the decision-making process. Health practitioners, for example, have experience of the actual context in which health interventions are provided, and the issues involved in micro-level priority setting. Patient groups, on the other hand, understand what it is like to actually live with conditions on a personal level – a perspective that may be overlooked when the prioritisation of conditions and treatments is discussed in the abstract. Other groups have knowledge of other factors that should be 24 accounted for in decision-making, such as different cultural perspectives or a thorough understanding of specific local issues.36 A final core rationale for increasing the involvement of stakeholders is based on legitimacy and the implementation of prioritisation decisions. The controversy that greeted the principles articulated by the Dutch Dunning Commission demonstrates the difficulty of implementing measures that do not enjoy public legitimacy. In contrast, the involvement of a wide cross-section of interested parties increases the likelihood that the decisions made by priority setting bodies will be accepted as reasonable. While this will partly be due to the ability of stakeholders to affect the decision-making process, involvement in decision-making can also lead to a greater appreciation of the issues involved in priority setting and a recognition of the need for compromises and making of difficult decisions.36 This legitimacy factor is particularly important in considering the involvement of health practitioners in priority setting. This is due to the fact that, as noted in the introduction, practitioners are themselves priority setting figures. Although their prioritisation capacity is partially limited by decisions at higher levels – such as the decision to exclude a treatment from funding – the role of health professionals in assessing conditions and prescribing interventions is a final “gate-keeping” role whereby access to health interventions is actually determined. This characteristic makes the assent of health practitioners vital in the establishment of effective prioritisation processes. Critical to this is the capacity of practitioners to subvert processes if they do not perceive them as legitimate. There is strong evidence, for example, that clinicians are willing to game waiting list systems they perceive as unfair, and assign patients a higher need for intervention than would generally be accepted in order to ensure earlier treatment.37 Similarly, the use of evidence-based medicine as an aid for prioritisation relies on health practitioners accepting the findings of relevant research – and there exists evidence that many health professionals place more trust in their own clinical experience and established tradition than scientific evidence.29 The clinical legitimacy of prioritisation systems and processes is thus of critical concern. Involving health practitioners in the development and operation of priority setting systems will not necessarily eliminate subversion of prioritisation processes. However, increasing their involvement does make it at least theoretically more likely that practitioners will see the resultant decisions and mechanisms as acceptable – both through a sense that the clinical viewpoint has been considered in their development, and via practitioners themselves becoming more aware of the pressures and issues involved in making prioritisation decisions. It is important to note, however, that while the involvement of stakeholders in priority setting is generally seen as positive, there remains debate over the issue. This is particularly the case with regard to the involvement of the public, with most objections to increasing involvement centring on the representativeness (or rather, the lack thereof) of those consulted, lack of specialised professional knowledge, and a potential descent into populist priority setting.38 Some theorists, particularly those who advocate a strongly technical and rationalist approach to prioritisation, also decry what they see as the essentially illegitimate involvement of sectional interest groups in decision-making processes.5 Perhaps the most interesting opposition to increased public involvement has come from the public themselves, with several studies of public preference returning findings that suggested public involvement in priority setting should be limited in comparison to those of clinical and managerial health 25 professionals (although results have varied significantly depending on how questions were phrased).36 In practice, however, these issues have been expressed in terms of the extent to which stakeholders should be involved, rather than attempts to eliminate their input completely. 5.2 How to involve stakeholders In terms of actually involving stakeholders in prioritisation, there exist two main approaches. The first of these is to use stakeholders to inform prioritisation decision-making. The opportunity to make comment on policy formulation is a fundamental principle of modern western democracy, and is commonly used to determine the values and principles that should underlie priority setting and to provide feedback on proposed processes. This can occur not only during the initial development of prioritisation processes, but also afterward in order to monitor changes in community values and preferences and obtain feedback on the operation of decision-making systems. Oregon provides an excellent example of this, with the Stateoperated Oregon Health Decisions undertaking biannual research to track the opinions of Oregon citizens. A wide variety of tools have been used for this form of consultation.38 Quantitative approaches such as telephone-based and questionnaire surveys have been a popular form of public consultation. Extensive use has also been made of more qualitative consultation methods. Such approaches allow a comprehensive examination of responses, and often provide a forum for two-way communication between decision-makers and stakeholders rather than the pure information collection of survey methods. The series of community meetings and hearings held by Oregon Health Decisions are a good example of this, as is the Dutch government’s publicity campaign to promote feedback on the report of the Dunning Committee. Focus groups have been used increasingly in this manner, as they avoid the selfselection biases of public meetings – although the limited number of people involved hampers their effectiveness at transmitting information from decision-makers to the public.6 One interesting example of this focus-group style of community consultation is that of Citizen Juries. Originating in the United States,xv and common both there and in Germany, recently this model has been exported to both the United Kingdom and Australia.39,40,41 The name of this approach stems from its similarity to that used in jury trials: a panel of usually between 12 to 16 members is chosen at randomxvi – usually through the local electoral roll, though other methods are sometimes used.41 This panel then sits for up to a week considering a single question under conditions modelled on those of a court case; for example, “witnesses” are called to give evidence and be cross-examined, and the jurors are expected to come to a consensus regarding their answer to the question, which is then presented to the body that has commissioned the jury. Whether qualitative or quantitative approaches are used, each of these forms of consultation provides important information that can be fed back to decision-makers and used in the development and operation of prioritisation systems. Importantly, however, this approach continues to position the public outside the actual process of setting priorities. Information is The term “Citizens Jury” has been trademarked by the Jefferson Center in the United States. Methodologically, Citizen Juries are not required to be statistically representative samples of a particular population. However, attempts are usually made to ensure that their makeup roughly reflects that of the local community. xv xvi 26 obtained from the public, patients, practitioners etc., but the responsibility for translating this information into practice still rests with a comparatively select group. The second form of stakeholder involvement differs from this in that it gives stakeholders an active role in the making of priority setting decisions. This form of involvement is far more controversial than the consultative role, as it involves stakeholders actively participating in the allocation of resources. Those theorists noted earlier who see public involvement as problematic, for example, may be willing to accept consultative involvement on the grounds that it constitutes a form of basic information-gathering. However, allowing those with an interest in the outcome of decisions a role in making those decisions is seen by such theorists as an example of defective policy processes.5 It has also been noted that involving stakeholders directly requires at least some transfer of authority and power to these groups, and this can lead to significant opposition from some traditional authority figures such as health professionals and administrators.42 Another point of view supports the active involvement of particular groups in priority setting, but argues that the types of groups involved should be limited. It has been argued, for example, that making effective prioritisation decisions requires significant clinical knowledge, and that therefore while health professionals should be involved in such processes other groups may legitimately be excluded on the grounds that they do not possess this knowledge.43 Although little research has been conducted on the extent to which this lack of knowledge is an issue in practice, at least one study has found that it does seem to present a significant problem – though one that can be overcome.44 As noted earlier, some research has indicated that this view may be shared by many members of the public itself, who feel that while it is important to gauge public opinion, the public in general do not need to be completely involved in the actual prioritisation process. Conversely, others have argued for the direct involvement of public and patient representatives in priority setting processes. Active public involvement in these decisions makes it more likely that the values and preferences of the public are actually incorporated into decision-making, and therefore enhances the legitimacy of the resulting priorities.42 This can include public representatives acting in a “watchdog” role, and ensuring that appropriate processes are followed and not undermined by the interests of other stakeholders. Similarly, involving patient groups directly in prioritisation decisions ensures that an appreciation of the actual experiences of those requiring interventions are included in decisions.44 Direct involvement is also the most effective way to promote understanding amongst stakeholders of the pressures involved in making prioritisation decisions. The implementation of this form of stakeholder involvement is significantly less common than the first form. Clinical guidelines require the extensive involvement of relevant health professionals if they are to possess any credibility, and therefore health professionals have been critical components in their development. In some jurisdictions the public may elect members of bodies responsible for meso-level decision-making (such as health boards), thus ensuring a level of direct input into such processes. Directors of medicine and nursing may play a similar role in representing the views of those health professionals in setting priorities within particular institutions. Finally, initiatives such as the Expert Patients Programme in 27 the UK enhance the involvement of patients in micro-layer decision-making regarding their own care.xvii 5.3 The NICE approach to stakeholder involvement The processes of the National Institute for Clinical Excellence in the UK provide a welldeveloped example of this second form of stakeholder involvement. The procedure whereby NICE formulates clinical guidelines and evaluates health interventions has been discussed above. The groups it concentrates on involving are health professionals, patients (ie; those whom the health intervention is intended to benefit) and their carers, and relevant commercial interests – and, in the case of clinical guidelines, the NHS.45,46 In the development of clinical guidelines, representatives of these interests are invited to register as formal stakeholders with NICE (in the case of health professionals, patients and carers this representation is achieved through national organisations). These representatives then provide comment on both the initial scope and two consultation drafts of the guideline. The Guidelines Advisory Panel acts as a watchdog to ensure that the comments made by stakeholders are given fair consideration by those developing the guideline.46 Furthermore, the Collaborating Centres responsible for determining the scope and developing guidelines consist of representatives of relevant health professions.xviii In Technology Assessment, the involvement of stakeholders works slightly differently. Initially, the procedure is the same as that for clinical guidelines, with representatives registering with NICE and providing commentary on the draft scope for the assessment. After this, however, the stakeholders, individuals from NICE co-ordinating the process, and the group undertaking the assessment, meet with each other to discuss issues involved in the assessment, and the stakeholders formally submit evidence to the Institute to support their position. Furthermore, once the assessment group has passed their report to the Appraisal Committee, not only is the report distributed for comment amongst stakeholders, but the Appraisal Committee meets and is attended by experts nominated by professional patient/carer organisations. What is particularly noteworthy is the importance given to the perspectives of patients and carers in both developing clinical guidelines and assessing technology – indeed, NICE has explicitly stated the importance of such views.46 Furthermore the Institute has recently appointed a Citizens’ Council (a form of permanent Citizens’ Jury meeting twice a year) in order to obtain public feedback on its decisions and “provide a backdrop against which NICE and the independent committees that advise it can develop their recommendations.”47 Although the Citizens’ Council is a purely consultative body, the bulk of NICE’s approach to stakeholder involvement is an example of actively incorporating stakeholder views into decision-making processes. With regard to clinical guidelines, as is usual practice these are developed by clinicians. Furthermore, although representatives of patients, carers and health professionals generally are technically only given a consultative role in both the assessment and guideline development processes, the extensive involvement of these groups – including xvii The Expert Patients Programme is an initiative implemented from 2002 by the National Health Service to promote people developing the skills to effectively self-manage their own chronic conditions. xviii In addition, the NCC for Mental Health also includes patient/carer representatives in the form MIND (the largest mental health advocacy organisation in England in Wales), Rethink (the former National Schizophrenia fellowship), and the Manic Depression Fellowship. 28 the aforementioned role of the Guidelines Advisory Panel in ensuring their comments are included – effectively give these groups a significant role in the actual development of the guidelines. Although it would be wrong to characterise this attempt to ensure patient involvement as wholly successful, the Institute has demonstrated a clear commitment to improving and enhancing the role of patients and carers. SUMMARY As the previous discussions indicate, there is perhaps one more dominant theme in international experiences and discussion of prioritisation: the on-going difficulty of implementing prioritisation systems in the health sector. Although there are certain dominant features of modern prioritisation systems – such as a concern with institutional rather than technical approaches in developing prioritisation processes, and an emphasis on evidencebased medicine and micro-level priority setting, no jurisdiction can claim to have successfully resolved the prioritisation debate. Although many jurisdictions have identified what values and principles should guide prioritisation, translating these into practice has proven both controversial and difficult. Similarly, while clinical guidelines have been promoted as an example of how clinical autonomy can be preserved within the development of explicit prioritisation processes, there is evidence that these guidelines are ignored in practice. The establishment of explicit prioritisation systems is therefore something of a thankless task for decision-makers. The National Institute for Clinical Excellence, for example, in many ways represents the leading edge of approaches to prioritisation. As an institution devoted to evidence based medicine, with extensive involvement of a range of stakeholders as an integral element of the formulation of its advice, and willingness to reflect and reform its own practices, NICE embodies the modern method of thinking about and approaching the rationing of access to health interventions. And yet there remain important questions and issues regarding its activities and decisions that make it impossible for the Institute to be classified as anything more than a qualified success.33,34,48 In summary, there can be said to exist a common international approach to priority setting – one emphasising the explicit identification of principles, the creation of robust processes rather than technical solutions, the need for continued clinical flexibility, the pursuit of evidence based medicine and increasing stakeholder involvement. The translation of this approach into practice is, however, far from complete. 29 SECTION TWO: THE NEW ZEALAND EXPERIENCE INTRODUCTION This section of this paper traces the development of prioritisation systems in New Zealand. It begins by discussing the pivotal role of the Core Services and National Health Committees in setting the groundwork for prioritisation systems in New Zealand. Following this, the different “layers” at which prioritisation decisions are made is used as a structure to explore experiences of priority-setting in the New Zealand health sector. It should be noted that although technology assessment is an important element of prioritisation systems, this discussion only touches on such assessment as it relates to other prioritisation processes. The National Health Committee is currently undertaking another project specifically focusing on issues surrounding the assessment of new health interventions in New Zealand, and substantive discussion of this area will be available in the report of this project. The purchaser-provider split and the origins of prioritisation debates As with other jurisdictions, the establishment of priorities for access to health interventions in New Zealand has historically been an implicit process. Although the fourth Labour government pursued several reviews of the health sector during the 1980s, 49 none of these led to significant alterations in how prioritisation decisions were made. This changed in 1990 when the incoming National government appointed a taskforce to undertake a major review of the sector, focussing on concrete proposals for restructuring the provision of health and disability support services. The resultant Green and White papers put forward six major recommendations,xix all but one of which were implemented to some degree. Using what has been described as a “big bang” approach to health sector reform,50 the government instituted a comprehensive package of structural changes that fundamentally altered the manner in which health services were offered in the New Zealand context. One of the most significant elements underlying these reforms was the institution of the “purchaser-provider split,” which involved the dramatic reconfiguration of meso-layer decision-making about health and disability services. Prior to the National government reforms, meso-layer decisions in New Zealand were made by fourteen Area Health Boards, each of which was responsible for providing health and disability support services throughout a particular geographic region.xx Area Health Boards received funding directly from the government on a population basis, allocated this to particular interventions, and then provided those interventions to people within their area. xix In addition to the establishment of Regional Health Authorities, Crown Health Enterprises and the Core Services Committee discussed above, the taskforce recommended the establishment of a Public Health Commission and accompanying Agency, transformation of the operationally-focussed Department of Health into a Ministry with primarily strategic and monitoring functions , and the introduction of alternative Health Care Plans to compete with RHAs for government funding. The last of these was the sole recommendation that was not implemented in some form, although the Public Health Agency was never established and the Public Health Commission was disbanded after less than two years of operation. xx These Boards were themselves the product of health sector restructuring by the fourth Labour government. 30 The reforms dramatically restructured this system. Firstly, administrative structures were reorganised and the localised Boards were replaced by four Regional Health Authorities (RHAs) responsible for far larger areas than the Health Boards.xxi The Authorities took over responsibility for administering the funding allocated by the government to support health and disability support services within their region. More fundamentally, however, the reforms split the previously integrated funding and provision role under which Area Health Boards had operated. Instead, the RHAs were expected to identify health needs within their areas and contract other bodies to provide interventions and services that would address those needs. Hospitals were commercialised into “Crown Health Enterprises” (CHEs),xxii and expected to both return a profit to the government and compete with private and not-for-profit providers of health services.51 OPENING THE DISCUSSION: THE CORE SERVICES COMMITTEE It was in the context of this dramatic restructuring that the issue of prioritisation was first publicly raised in New Zealand. One of the recommendations of the taskforce was the establishment of the National Advisory Committee on Core Health and Disability Support Services, also known as the Core Services Committee (CSC). The CSC was established in 1992 “to advise the Government on core health and disability support services to which the public should have access on fair terms.”52,xxiii In other words, and in keeping with thendominant thinking, the committee was initially positioned to follow the lead of the State of Oregon in the USA and determine what health services should be funded by the government. One of the key reasons for establishing the Core Services Committee as an independent committee, rather than giving this role to the Ministry of Health, was to fully engage the public in the priority setting process.53 To this end, the CSC undertook extensive periods of consultation using a wide variety of methods.54 Beyond this, however, the committee’s position outside traditional health sector structures, the relatively wide membership of the committee, and the approach taken to consulting with the public, represented an attempt not only to ascertain what priorities stakeholders saw as important, but also to improve public understanding of the pressures and issues that were facing decision-makers in the health sector. For example, the committee’s Best of Health series of documents included several example funding scenarios to illustrate the types of contexts in which prioritisation documents are made.xxiv The establishment of the committee in New Zealand was driven by many of the same pressures driving the institution of explicit prioritisation processes elsewhere, with the key xxi The four RHAs were North Health (Northland and Auckland), Midland Health (the central North Island), Central Health (the Lower North Island, the Chatham Islands, Nelson and Marlborough) and Southern Regional Health (the South Island except Nelson and Marlborough, and Stewart Island). xxii CHEs were later renamed hospitals by the National-New Zealand First Coalition Government of the late 1990s. xxiii Importantly, the committee’s brief at this time covered only personal health and disability support. Public health issues were the responsibility of the Public Health Commission. xxiv This series consists of The Best of Health: Deciding on the health services we value most (1992), The Best of Health 2: How we decide on the health and disability support services we value most (1993), and, published by the renamed National Health Committee, The Best of Health 3: Are we doing the right things and are we doing those things right? (1997). 31 driver being a belief that without some process to limit the services that would be funded, an inevitable expansion in needed and available services would lead to a major crisis in health funding: By defining core health services, we can get better value for scarce resources and seek to limit the growth of medical expenditure. If we do not do this, new medical technology and pressures arising from demographic change will overwhelm our health system.55 Initially, the committee considered three broad methods through which a “core services” approach to prioritisation could be adopted, the first two of which were variants on the same “general list” method and could theoretically have been combined for greater control over meso-layer prioritisation: 1. Positive general listing: In this approach, the committee would define a core list of specific groups of interventions that all meso-layer decision-makers (ie; the Regional Health Authorities) would be required to fund. 2. Negative general listing: In this approach, the committee would define a list of specific interventions that meso-layer decision-makers would be prevented from funding through public resources. 3. Priority-listing: In this approach, the committee would adopt the same model as Oregon and rank interventions on a priority basis. This list would be developed along similar lines to those advocated by the Dunning Committee in the Netherlands, with care being ranked according to its effectiveness, efficiency, necessity from a community perspective, and ability to be left to individual responsibility. The level of funding devoted to the health budget would then determine the particular interventions that could receive public funding. After fully considering these options and in light of consultation with health practitioners, the public and other stakeholders, however, the CSC rejected all three of these methods – primarily due to the inability of these approaches to take account of the specific clinical contexts in which interventions may be required. Instead, the committee focussed its attention on examining the principles that should guide prioritisation decisions made by others in the sector. After further consultation, the CSC – by now renamed the National Health Committee (NHC) – developed the following principles:56 Effectiveness – a funded intervention should show good evidence of benefit. Equity – funding an intervention should constitute a fair use of public resources. Acceptability – offering an intervention should be consistent with community values. Efficiency – funding an intervention should offer good value for money. These four principles, with some modification, have underpinned all subsequent work on prioritisation in New Zealand. The National Health Committee In 1996 the Core Services Committee became the National Advisory Committee on Health and Disability, usually referred to as the National Health Committee. This reflected a widening in the role of the committee – most clearly in the expansion of its remit to cover public health issues. This second role eventually led to the establishment of the Public Health Advisory Committee (PHAC) as a permanent subcommittee of the NHC in 2001. 32 Around this time, the committee also refocused the scope of its attention on prioritisation. As noted earlier, the principles to guide prioritisation decisions were published under the auspices of the NHC rather than the CSC. Since then, however, the NHC’s work on prioritisation has largely concentrated on the micro-layer of prioritisation decision-making. This has primarily involved work on developing clinical guidelines and Clinical Priority Access Criteria.57 The committee has also provided commentary on prioritisation work undertaken by other organisations. MACRO LAYER PRIORITISATION Since the Core Service’s Committee rejection of exclusion processes, there has been little attempt to develop explicit prioritisation processes at the macro-layer of the New Zealand health sector. It is important to note that priorities are still set at this level – for example, the Government-set Service Coverage Schedule (SCS) delineates the particular mix of services that must be provided by District Health Boards (DHBs).xxv Similarly, strategic documents such as the New Zealand Health Strategy and the Mäori Health Strategy: He Korowai Oranga also set priorities to guide funding decisions. These strategic documents also influence prioritisation decisions at the macro-layer by influencing where funding is allocated within the overall health budget. It is therefore fair to say that the first element of an explicit prioritisation system – identifying the values and principles that should underlie decisions – does exist at this level. However, the process whereby these priorities lead to the specific macro funding decisions that are made remains implicit. A few exclusions have been established at the macro-layer – most notably in screening for particular conditions. In the main, however, these are comparatively minor and marginal in nature and controversy continues to surround many of them. This is in line with international experiences, where attempts to exclude services from funding have generally (the American State of Oregon being the exception) proven very difficult to implement and have been implemented only in very limited forms. The lack of explicit methods of priority setting at the macro-layer is also in line with dominant international experience. There is, however, one part of macro-layer decision-making where these general rules do not hold true: Pharmac. Pharmac: an international anomaly Established in 1993, Pharmac is a national body that oversees the funding of pharmaceuticals in New Zealand. Initially, the body was set up as a shared agency under the Regional Health Authorities and later under the Health Funding Authority (see below), before being established as a fully independent crown entity in 2000. Pharmac was established to “secure for eligible people in need of pharmaceuticals the best health outcomes that are reasonably achievable from pharmaceuticals treatment and from xxv To avoid confusion it should be noted that this does not constitute an exclusion-based approach to funding, as it does not prevent resources being allocated to other interventions. 33 within the amount of funding provided,”58 and to this end it manages the funding allocated by the government for community purchase of pharmaceuticals. The role of the agency is therefore to determine what pharmaceuticals should receive public funding, the level of subsidy they receive, and any guidelines or conditions relating to their prescription. xxvi These details are recorded on the Pharmaceutical Schedule. Pharmac’s decisions are subject both to internal appeal processes and judicial review. The agency also maintains a Pharmacology and Therapeutics Advisory Committee (PTAC), consisting of (primarily medical) health practitioners, to provide expert advice. Pharmac is not, however, bound to accept the recommendations of this committee. The Pharmac approach to prioritisation emphasises cost-effectiveness, primarily using CostUtility Analysis.xxvii The agency does include reference to wider principles in its decisionmaking processes, such as government-established priorities for health funding. However, Pharmac has wide discretion in choosing how to apply these criteria, with the agency “giving such weight to each criterion as Pharmac considers appropriate,”58 and providing little public documentary evidence as to how it these factors influence its decision-making.53 In wider terms, Pharmac represents something of an anomaly both in New Zealand and internationally with regard to prioritisation decisions. In particular, the agency appears to have experienced few of the problems that have faced other institutions in setting priorities, notably surrounding restricting investments in existing interventions. Indeed, the institution is arguably the most successful prioritisation body in New Zealand – at least from a purely “cost-containment” point of view. Between 1993 and 2001 the body listed 630 new or enhanced products, widened the conditions for access for another 137, limited access to 36, and de-listed 668.59 Similarly, where other countries have experienced rapid growth in public expenditure on pharmaceuticals, New Zealand’s overall expenditure has actually declined since 1998. Furthermore, Pharmac continues to make these decisions through a strongly technical approach. The spread of institutional approaches to prioritisation has largely bypassed the agency, with decisions continuing to be made primarily with reference to CostUtility Analysis.53 There has been little research, however, into possible reasons for Pharmac’s unusual success. Some have suggested that it may be due to the availability of comprehensive data on costs and benefits of pharmaceuticals compared to interventions in other parts of the health sector.60 This is partly supported by the near-perfect record of Pharmac in defending its decisions when they have been subjected to judicial review, which suggests that its decisionmaking processes are robust and defensible. However, the UK’s National Institute of Clinical Excellence has had far less success in a similar area, suggesting that Pharmac’s success is likely due to something more than simply the quality of information available. xxvi This situation, in which Pharmac acts as the sole purchaser of pharmaceuticals, is referred to as monopsony. Just as in a monopoly, where there is only a single seller within a “normal” market, prices will theoretically be higher than otherwise, a monopsonistic situation is intended to ensure that prices are lower than otherwise. xxvii As noted earlier, Cost Utility Analysis is a form of economic evaluation in which the likely benefit of providing an intervention is measured in terms of the Quality-Adjusted Life Years gained as a result of that intervention, and this benefit compared against the cost of providing that intervention. This is intended to give an idea of the efficiency of an intervention: lower costs per QALY gained translates to greater efficiency 34 MICRO LAYER PRIORITISATION AND EVIDENCE BASED MEDICINE In contrast to macro-layer prioritisation, extensive work has been undertaken in New Zealand on developing processes to aid in prioritisation decisions at the micro-layer. This has primarily involved the development of clinical guidelines and Clinical Priority Assessment Criteria. Clinical guidelines and the New Zealand Guidelines Group Clinical guidelines are a well-established element of New Zealand’s health sector. The production of these guidelines is undertaken by a wide variety of agencies and bodies; the National Health Committee, the Accident Compensation Corporation, non-government organisations and the Ministry of Health amongst others have developed guidelines specifically for the New Zealand context. Particular attention should be paid here to the position of the New Zealand Guidelines Group (NZGG). The Group was originally established in 1996 under the auspices of the NHC, as a network of experts interested in the development of such guidelines. The activities of this network underpinned the NHC’s early work on guideline development. In 1999, the NZGG left the National Health Committee and established itself as an independent not-for-profit organisation. The Group now defines its role as being to lead “a movement towards the delivery of high quality health and disability services throughout New Zealand through a change in culture based on evidence and effectiveness.”61 This involves two core activities. In the first instance, the NZGG develops clinical guidelines itself – for example, the Group has recently produced guidelines for the management of Type 2 Diabetes, the assessment and management of people at risk of suicide, and the assessment and management of cardiovascular risk. Perhaps more important than this function, however, is the role of the NZGG in facilitating the use of these guidelines. Firstly, the Group maintains a database of guidelines developed in New Zealand. This allows guidelines to be easily accessed by health professionals. Secondly, the NZGG undertakes activities to promote the visibility and use of guidelines through, for example, co-sponsoring publication of the monthly Evidence Bulletin and holding conferences on guideline use. Finally, the Group provides advice and training both for the practitioners at whom guidelines are aimed, and for those involved in developing guidelines. The formulation and dissemination of clinical guidelines is a well-established feature of New Zealand’s health sector. However, there has been comparatively little research into the actual use of guidelines in New Zealand’s clinical settings. This is an important issue as guidelines can clearly only have impact as prioritisation tools if they are actually used by practitioners. Internationally, this is a significant problem – several studies have shown that even when guidelines are viewed positively their actual use by health practitioners often remains low. 30 A recent review by the New Zealand Guidelines Group of a trial on adapting guidelines to local conditions has identified the existence of some important barriers to the uptake of such guidelines by clinicians.62 However, further research on issues surrounding the uptake of guidelines is clearly justified. 35 Clinical Priority Access Criteria The second main process that guides prioritisation at the micro-layer in New Zealand is the use of Clinical Priority Access Criteria (CPAC). Whereas clinical guidelines relate to clinical decisions to prescribe an intervention, CPAC influence when a given patient receives that intervention – their position within the relevant booking system for elective services.xxviii All providers of surgical services require some method of planning the allocation of resources for providing those services. In this context, many theorists and decision-makers make a distinction between “waiting lists” and “booking systems”. In this case, “waiting lists” refers to situations in which the scheduling of interventions for patients occurs primarily on a “firstcome, first-served” basis, with the only difference between individuals being a differentiation between “urgent”, “semi-urgent” and “routine” need for surgery. While such an approach can be appropriate in some circumstances, such systems have been criticised as open to abuse and being unacceptably vague about when and on what basis a patient will actually receive an intervention.63 In contrast, booking systems of the type used in New Zealand are intended to provide a clear guarantee about when a particular patient will receive surgery. As figure one below illustrates, these systems incorporate the extensive use of guidelines to manage demand, with the intention that surgery can then definitely occur within a specified period. Figure One: Intended operation of New Zealand’s booking system for elective surgeryxxix Patient assessed need Person develops clinical symptoms and/or signs Surgical management guidelines GP referral guidelines Consult GP x GP not consulted x Referral not indicated – conservative management Refer to surgeon x Surgery not indicated – return to GP care for conservative management Priority system threshold Surgery indicated x Above threshold – booked for surgery within 6 months Below threshold – return to GP care for conservative management and review The transition to a booking system was one of the initial recommendations of the Core Services Committee. The key foundation of this process was the development of CPAC – points-based systems in which the priority for offering a particular surgical intervention is scored using a formula that weights a variety of biomedical and social factors such as the severity of the condition, capacity to benefit from the intervention, and the effect of the intervention on a person’s independence.49 As noted in the above figure, those who achieve a It should be noted that in this specific context, the term “intervention” refers only to surgical intervention. Derived from Figure 6.2 in Roake J, “Managing Waiting Lists”. In Gauld R, ed. Continuity amid chaos: health care management and delivery in New Zealand. Dunedin: University of Otago Press; 2003:107-121. xxviii xxix 36 particular minimum score are guaranteed an intervention within six months, while those who fail to meet this level are referred back to their general practitioner for ongoing management. Since 1996, a wide variety of CPAC have been developed. Initial work in this area involved bringing together specialist physicians in working parties that then developed specific national Criteria for individual interventions that were both high-cost and constituted the majority of interventions on waiting lists. These first CPAC have been recognised as representing the leading edge of international criteria development.63 In 1998, however, the Health Funding Authority (see below) made the full introduction of booking and scoring systems a requirement for the receipt of funding for elective surgery.64 As a result, hospitals were faced with the need for a far greater range of Criteria than the intervention-specific forms that had been developed up to that point. This need in turn led to the creation of locally developed generic systems, based on the general principles underlying previous criteria, that prioritised across whole surgical specialties. In contrast to the initial CPAC, these generic systems were the subject of harsh criticism. The lack of piloting and pre-implementation assessment of the generic criteria was held to be a particular weakness, as was the lack of consistency between regions in terms of both scoring systems and thresholds for treatments – one regional ethics committee deemed the entire process for developing Criteria in their area to be fundamentally flawed.49,64 As a result of these issues, the National Waiting Times Project (and later the Elective Services Group) began the development of standardised, national, generic criteria to bring a level of consistency and ensure quality in the development of CPAC. Ultimately, however, the overall effects (positive or negative) of establishing booking systems and CPAC are unclear. In the first instance there exists little evidence that use of scoring systems, even those New Zealand examples considered world leaders, accurately translates into prioritising surgery for those with the most potential to benefit from it – the principle which is intended to underlie New Zealand’s booking system.63,64,65 Furthermore, while numbers waiting for surgery may have decreased since the introduction of CPAC, the absence of a substantive audit of processes and procedures for waiting list management prior to their introduction, and the concurrent implementation of other measures to reduce waiting times, make it difficult to distinguish what contribution may have specifically been made by scoring systems.64 MESO-LAYER PRIORITISATION As noted earlier, the Core Services and National Health Committees both shied away from placing tight restrictions on the authority of meso-layer decision-makers. While this has given significant freedom to meso-layer decision-makers (at least in theory), it has also led to significant problems. To a large extent this is because it is at the middle layer where the pressures of prioritisation can become most apparent and controversial. It is the macro-layer that controls the ability of these decision-makers to supply interventions, and the micro-layer that controls demand for interventions. Decision-makers here can therefore become the proverbial meat in the sandwich between restrictions from above and demands from below. 37 The Regional Health Authorities (1997 – 1998) Once the NHC had developed its key principles, the question turned to how these should be realised in practice. Responsibility for this was given to the Regional Health Authorities, with the NHC intending to maintain a watching brief on prioritisation issues. The majority of work on prioritisation by the Authorities was undertaken by the Midland Health RHA, which had responsibility for the central North Island. Using the National Health Committee’s work as a base, Midland Health developed five principles to underlie priority setting decisions:66 Effectiveness: the ability of an intervention to provide benefit. Equity: the need to ensure equality of opportunity for access to health interventions for groups and/or individuals with similar levels of need. Mäori Health: recognition of the Crown’s objectives for Mäori health and its obligations under the Treaty of Waitangi. Acceptability: consistency with the needs and values of communities. Efficiency: value for money. However, before this framework could be fully developed and implemented, the Regional Health Authorities were dissolved in favour of the establishment of a single national Health Funding Authority (HFA). The Health Funding Authority (1998 – 2000) The Health Funding Authority represented the centralisation of meso-layer decision-making in New Zealand’s health sector. In contrast to both the previous Area Health Boards and RHAs, the HFA brought responsibility for managing the funding of health interventions to a national level – although the Authority did maintain four regional offices to administer funding within those areas.xxx As the body responsible for meso-layer decision-making, the HFA also took up responsibility for developing a process for priority setting at this layer. Deciding not to reinvent the wheel, the Authority took as its basis the work previously being undertaken by Midland Health. The principles identified by that RHA were taken up, albeit with the renaming of the “efficiency” principle as “cost”, and the Authority began development of a process whereby these principles could be used to guide funding allocation. The HFA’s approach was based on the use of Cost-Utility Analysis. The Authority recognised that this approach would be both problematic and controversial as a method of prioritising between interventions, and its emphasis on the principles of acceptability, Mäori health, and equity, along with the particular process it adopted, was an attempt to deal with this.60 In practice, however, the Authority was concerned primarily about methodological issues associated with the use of CUA – exactly how these other principles would relate to the measurement of QALYs was unclear.53 In 1999, the Ministry of Health invited the National Health Committee to review the HFA’s proposed process. The committee in turn commissioned a formal evaluation of the process. xxx These regional offices were based on the previous RHA regions. 38 The resulting report, while supporting the underlying aim of the Authority, identified significant concerns with the HFA’s approach.13 These included: The manner in which the principles were to be interpreted in practice. The tools used to quantify effectiveness. The inability of CUA to take account of particular important health outcomes. The unavailability of important relevant information for many services The lack of clarity over how the HFA’s process would relate to decisions made at other levels of the health sector. The cost and impact of the prioritisation process itself. Consequently, the NHC recommended that the HFA exercise caution in continuing its prioritisation work and the Authority began a series of pilots to further refine the process. Before significant further work could occur, however, the accession of a Labour-Alliance government in late 1999 brought a further (and so far final) period of meso-layer restructuring. The present picture: District Health Boards and devolution As part of a decision to largely eliminate the purchaser-provider split, the HFA was incorporated into the Ministry of Health, and 21 District Health Boards (DHBs) established to both administer Crown funding and actually provide most health services within a particular region. The Ministry of Health gained responsibility for directly purchasing services in a small number of specific areas where it was felt that a national focus on purchasing was desirable, although some of these areas have since been devolved to DHBs.xxxi Currently, meso-layer decision-making in New Zealand is largely the responsibility of the DHBs, with the Ministry retaining capacity in some specific areas. As a result, prioritisation at this layer is far from nationally consistent. Each DHB and relevant part of the Ministry has developed their own process for making prioritisation decisions, and these vary notably from each other. Although there are some common themes – most notably in the use of the NHC/RHA/HFA principles in defining the core basis on which priority setting decisions should be made – the specific processes and principles that are used vary widely.60 In the main, however, Boards appear to be taking a broadly institutional approach to setting priorities inasmuch as their processes emphasise discussion, with tools such as Cost-Utility Analysis being used as simply methods of measuring one aspect of decision-making: It appears that many DHBs use consensual decision-making as a means of deciding, on the basis of information provided on the principles, which proposals go forward and which proposals do not.60 Importantly, however, meso-layer prioritisation decisions are influenced by prioritisation decisions made at the macro-layer. Firstly, as noted earlier, there exist several national strategic documents that outline priorities for the health sector as a whole. These include the xxxi Currently the two main areas in which the Ministry directly purchases services are public health, and disability support services for people under 65. 39 New Zealand Health Strategy, the New Zealand Disability Strategy, xxxii the Mäori Health Strategy: He Korowai Oranga, the Pacific Health and Disability Action Plan and the Primary Health Care Strategy. These strategies set out particular concepts and areas that meso-layer bodies must account for when prioritising. In effect, compliance with these documents acts as another overarching principle that guides prioritisation processes at the meso-layer. In addition to these documents, the macro-layer also exercises more focussed control over meso-layer prioritisation decisions. In the first instance, the Ministry’s Service Coverage Schedule (SCS) details the general types of services DHBs must ensure are available within their region. The Schedule is, with a few exceptions, not a specific list of the particular interventions that Boards are required to offer. Instead it lists general areas and types of services, such as “hospital specialist medical and surgical services” within the area of renal medicine.67 The existence of the schedule is intended to ensure that there exists a minimum and consistent range of interventions offered to people throughout New Zealand.xxxiii Outside these measures, a variety of macro-layer operational documents outline what sort of characteristics must be displayed by the prioritisation processes used by meso-layer decisionmakers. For example, the Operational Policy Framework requires DHBs to use a principlebased framework, involve Mäori and the community at large, and maintain clear records of the process that was followed in making prioritisation decisions.68 These documents do not actually affect prioritisation by DHBs and the Ministry, but instead influence the systems such decision-makers must use to make these decisions. Finally, the government’s annual Budget regularly links funding within Vote: Health to specific initiatives. These ring-fenced funds can be reserved for particular services (such as hip operations), areas within the overall health sector (such as public health), or measures to address the needs of particular populations. Such resources can only be accessed to provide the specific services and initiatives for which such funding is made available. Figure 2 overleaf demonstrates how the current different layers of prioritisation in New Zealand’s health sector are intended to interact.xxxiv xxxii The New Zealand Disability Strategy does not focus only on health services. The Strategy sets out objectives for all sectors, including aims relating to health, education, employment and human rights. xxxiii Ironically, it could be argued that this represents a form of the “positive listing” approach to prioritisation originally discarded by the Core Services Committee. xxxiv Note that this diagram is focussed on structures for providing services. It does not include Pharmac – a macro-layer body – or the New Zealand Guidelines Group which influences behaviour at the micro-layer. 40 Figure 2: Levels of priority setting in New Zealandxxxv Government decides its investment priorities over all spending portfolios, and allocates funding to Vote Health. Minister of Health (Ministry of Health as Minister’s agent) decides priorities for the health sector (for example, via the New Zealand Health Strategy) and sets the parameters for decision making through, for example, the letter of Ministerial expectations, indicators of DHB performance, the Service Coverage Schedule (SCS), the Crown Funding Agreement (CFA) and determining how funding is to be split. Funding held centrally by the Ministry to contract provision of certain services. Funding devolved to DHBs to contract provision of most services Ministry determines broad service priorities that match coverage outlined in the SCS through mix of government strategy documents, assessments from service directorates (including funder and provider consultation) – e.g. ‘public health programmes’. DHB determines broad service priorities that match coverage outlined in the SCS and CFA through mix of government strategy documents, health needs assessment and community consultation – e.g. ‘child health’, diabetes. Ministry determines priorities within ‘public health services’ – e.g. screening services. DHB determines priorities within ‘child health services’ – e.g. bedwetting alarms. DHB determines priorities within ‘diabetes services.’ Ministry determines high-need patients (e.g. through access criteria) for screening services. DHB determines highneed patients (e.g. through access criteria) for bedwetting alarms. Health practitioners make clinical decisions and apply access criteria. Health practitioners make clinical decisions and apply access criteria. xxxv Derived from Figure 1 in Ministry of Health. Current prioritisation processes in New Zealand. Wellington: Ministry of Health, 2003. 41 Ongoing issues at the meso-layer Recent research by the Ministry of Health has identified the existence of several key difficulties that DHBs feel are hindering their ability to make effective prioritisation decisions.xxxvi The barriers identified by Boards can be grouped into four major factors. Staff capacity Firstly, many Boards feel that their ability to make effective prioritisation decisions is limited by available staff capacity. In the first instance, this simply refers to the number of staff available to undertake necessary analysis. Prioritisation is a time-consuming and complex process even for larger DHBs, and this becomes even more of an issue for small Boards. The planning and funding activities expected of each Board are the same regardless of size and capacity, and consequently smaller Boards with comparatively smaller staff are required to undertake the same functions as larger DHBs without the personnel to support this. Furthermore, DHBs have identified a lack of individuals with a strong understanding of prioritisation processes and methodologies as a key hindrance to effective decision-making. This is the case not only with regard to analysts, but also in terms of Board members who are charged with interpreting information provided by analysts and making decisions on that basis. Once again, this may be less of an issue with larger Boards who may have the ability to draw on a larger pool of staff and Board members with previous experience in the HFA or with general priority setting expertise. Political factors A second major barrier to prioritisation relates to political factors surrounding such decisions. DHBs have noted that the communities they serve often do not appreciate the pressures and constraints under which they operate, and that stakeholders often do not accept the need for prioritisation decisions to be made – especially around disinvestment in existing services. This in turn makes priority setting difficult, as the financial realities of operating a DHB must be balanced with the beliefs and desires of the local community, clinicians, and macro-layer decision-makers. It is unlikely, however, that this problem will be easily overcome – if, indeed, it can be. As noted earlier in this paper, prioritisation is an innately contestable and controversial process that many have argued can have no “correct” conclusion. In this context, it is likely that there will always exist at least some dissatisfaction with decisions made by Boards. Increasing involvement of clinicians, the general public and other stakeholders in the prioritisation process may lessen this, but it is likely that setting priorities will always be subject to political reality. xxxvi The information in this section is, unless other noted, drawn from Ministry of Health. Current prioritisation processes in New Zealand. Wellington: Ministry of Health, 2003. 42 Macro-layer constraints The third key barrier to prioritisation relates to constraints on prioritisation decisions introduced at the macro-layer. These constraints can be divided into two groups. Firstly, there exist the overall levels of funding allocated to DHBs. Boards have argued that current levels of funding – especially, under the population-based funding formula, funding for smaller Boards – simply does not provide enough resources to effectively prioritise funding on new interventions. At the same time this makes prioritisation in general more difficult and pressured, as attempts to reduce deficits require disinvestment in services. This budgetary constraint is seen as particularly critical given the second macro-layer factor: ring-fencing and tagging of funding, and the rigidity of documents such as the Service Coverage Schedule and Operational Services Framework. It has been argued that not only does this reduce the ability of DHBs to be flexible and reallocate funding in an effective manner, but when new funding is allocated it is often linked to specific services that may not be related to areas of particular concern for a specific Board. Although not often explicitly framed as such, this discontent is effectively a restatement of the core services debate. This debate is essentially over how control over access to interventions should be distributed between macro-, meso-, and micro-layers. Critics of current arrangements claim that, while DHBs may ostensibly have significant decisionmaking power – and therefore have commensurately high levels of accountability regarding the outcomes of allocating their resources – in practice, the macro-layer exercises significant constraints over their ability to make decisions. The net result of this is the majority of DHB expenditure is tied up in providing a large de facto “core”, and practical Board autonomy is confined to a far smaller group of marginal services. This, it has been argued, leads to a perverse situation in which Boards are held publicly and legally accountable for achieving particular health outcomes, and yet are constrained in their ability to achieve those outcomes by macro-layer decisions regarding required outputs. Availability of information The final key factor inhibiting Boards’ ability to prioritise effectively relates to the information that underpins prioritisation decisions. At a fundamental level, this relates to the identification of candidates for disinvestment or investment. Beyond this, however, some Boards have identified a perceived lack of information relating to the costs and benefits of particular services. This relates both to the availability of the information itself and also exactly how these costs and benefits should be assessed. This, in turn, feeds into the previously identified barrier of staff capacity, as analysts and Boards face significant obstacles in carrying out analysis and making decisions on the basis of such analyses. As with political barriers, however, this is a complex issue and one that may not be amenable to easy answers. For example, arguments over how the “benefit” of an intervention can and should be measured have been conducted amongst and between theorists, advocacy groups and policy-makers for decades – the “QALY” debate being perhaps the most visible of these. 43 Consistency of approaches In addition to these factors, there also exists a general level of dissatisfaction amongst some with regard to the overall picture of approaches to prioritisation. There currently exists no unified approach to prioritisation. As noted earlier in this paper, each DHB has developed a different process for making prioritisation decisions, and although the principles developed by the NHC and refined through the RHAs and HFA usually provide the core to these processes, the full range of decision-making criteria and the precise processes for making prioritisation decisions varies significantly. On top of this, the Ministry of Health operates several different prioritisation processes to guide its own funding decisions. This highly differentiated situation is defensible on the grounds that prioritisation processes need to take account of the particular contexts within which they operate. Different DHBs operate within different environments and serve different populations, while different Directorates in the Ministry of Health deal with different forms of “health”. A rigid “onesize-fits-all” approach would likely fail to reflect these variations. However, this does not mean that the current level of difference is appropriate, and there exists a clear desire for an increase in consistency between prioritisation decision-makers. Consequently, work has begun on developing a unified approach to making prioritisation decisions that could be used by all meso-layer decision-makers. A joint project of several DHBs and the Ministry, with input from both policy-makers and the wider health sector, has been looking at this issues and led to the development of a framework to apply in making prioritisation decisions. This framework attempts to balance the previously noted desire for increased coherency with the need for processes to be adaptable to local conditions. At the time of writing, the National Health Committee is evaluating the experience of those decision-makers involved in piloting the framework. SUMMARY The state of prioritisation in the New Zealand health system differs significantly at all three layers of decision-making. Although there exists a strong theoretical commitment to making prioritisation decisions explicit, the extent to which this has been translated into practice varies markedly between layers. At the macro-layer, prioritisation remains essentially an implicit process. The health system does clearly define priorities at this layer, both through strategic documents such as the New Zealand Health Strategy and He Korowai Oranga, and the operational requirements placed on meso-layer decision-makers. These documents do influence central government funding of the health system – if they do not affect the overall allocation of funding to Vote Health they certainly underlie the allocation of resources within that Vote. However, the process whereby these principles are translated into practice is not clear. Although funding may be allocated on the basis of these principles, there is no clearly-defined process that sets out how this is undertaken. At the micro-layer, in contrast, considerable attention has been paid to prioritisation in the form of the development of clinical guidelines and access criteria. New Zealand has been recognised as a world leader in these areas, and as a country is arguably one of the leaders in implementing evidence-based approaches to clinical decision-making. Important issues still 44 exist at this level regarding the effectiveness of these processes. However, these questions and difficulties are less a product of New Zealand’s specific implementation than underlying dilemmas surrounding decision-making at the clinical level. Finally, meso-layer prioritisation occupies an uncertain middle ground. Although discussion of priority setting at this level has been near-constant since the early 1990s, it has also been a victim of the continual restructuring that plagued the New Zealand health sector throughout the decade. Neither the RHAs nor the HFA remained in existence long enough to progress far beyond their initial work, and DHBs have spent the years since their establishment primarily building their own capability and capacity to maintain existing service arrangements. As a result, while meso-layer priority setting is largely explicit there exist a plethora of different systems and processes throughout the country, and DHBs at least believe that there exist significant barriers to making effective prioritisation decisions. In this context, the previously mentioned work of a joint Ministry-DHB working group to develop a unified, yet flexible and locally-adaptable, framework for making prioritisation decisions has significant potential benefit. 45 APPENDIX: DEVELOPMENT AND ASSESSMENT PROCESSES OF THE NATIONAL INSTITUTE FOR CLINICAL EFFECTIVENESS Guideline Development The process for establishing clinical guidelines begins with the Department of Health (DoH) and the National Assembly for Wales (NAW), with these two bodies jointly deciding to refer a particular topic to the Institute. In making this decision, the DoH and NAW are required to have regard to whether or not developing such guidelines could:46 - improve health; reduce costs to the NHS; link with a technology appraisal (see below) being carried out by NICE; link with Government priorities for health; and help to reduce variations in clinical practice or care. The guidelines themselves are developed through one of six National Collaborating Centres (NCCs), each of which specialises in a particular area of health services: Acute Care, Chronic Conditions, Mental Health, Nursing and Supportive Care, Primary Care, and Women and Children’s Health. These are organisational, rather than geographic, centres and include a variety of professional, academic, and other relevant bodies.xxxvii Once approached to develop a guideline, the members of a particular NCC establish a Guidelines Development Group (GDG) which has responsibility for actually formulating the guidelines on behalf of the Centre. In addition, NICE establishes a Guidelines Advisory Committee Panel (GAC Panel) for each individual guideline. This panel includes a variety of members, including an adviser on patient and carer issues and an economic adviser, and is responsible for overseeing and formally approving the guidelines developed by the GDG. Although there is no set method for actually formulating the guidelines, the overall process entails a series of phases through which the Centre must proceed. xxxviii Firstly, the Centre develops a scope which covers the patient groups to whom the guideline will apply, the particular healthcare setting that the guideline will focus on (for example, general practice or a hospital setting), the use of which treatments and/or approaches will be considered under the guideline, and the manner in which the Centre will assess the clinical and cost effectiveness of these treatments and/or approaches.46 After consultation on this scope the NCC develops a workplan for developing the guideline and, once NICE agrees to this plan, proceeds with developing the guideline. This development proceeds through two phases in which the NCC circulates draft guidelines for xxxvii The NCC for Acute Conditions, for example, includes the Royal College of Surgeons, the Faculty of Dental Surgery, the Royal College of Anaesthetists, and the Royal College of Ophthalmologists. The NCC for Primary Care, on the other hand, consists of the Royal College of General Practitioners, Leicester University’s Clinical Governance Research and Development Unit, the Community Practitioners and Health Visitors Association, Sheffield University’s School of Health and Related Research, and the Royal Pharmaceutical Society of Great Britain. xxxviii Full descriptions of this process can be found in the NICE publications The Guideline Development Process – Information for the Public and the NHS, The Guideline Development Process – Information for Stakeholders and The Guideline Development Process – Information for National Collaborating Centres and Guideline Development Groups. 46 comment from both stakeholders and the GAC Panel before the presentation of the final guidelines for approval by the GAC Panel and NICE itself. Although there is no set process (apart from the aforementioned phases) that the Centre must follow in determining guidelines, certain principles are expected to underlie their formulation. They should:46 - aim to improve the quality of clinical care; address the clinical effectiveness of treatments or management approaches; address the cost effectiveness of treatments or management approaches; be advisory; be developed through a process that considers all who might be affected by the guideline; be based on the best possible research evidence and professional consensus; be developed using methods that command the respect of patients, the NHS and NHS stakeholders; be developed with the involvement of patients and healthcare professionals; set out the clinical care that might reasonably be expected throughout the NHS in England and Wales. As can be seen, the majority of these principles relate to how such guidelines should be formulated. In terms of guiding decision-making, the only principles underlying the guidelines themselves are those of clinical effectiveness and cost effectiveness. Technology Assessment NICE’s method of assessing new health interventions is similar to that used to develop guidelines. In the first instance, the DoH and the NAW request that a given health technology be appraised. “Technology” is defined broadly, and includes pharmaceuticals, medical devices, diagnostic techniques, surgical procedures, “other therapeutic interventions”, and methods of health promotion.45 In making this decision, the DoH and NAW are assisted by the National Horizon Scanning Centre and National Prescribing Centre, both of whom provide advice on new or emerging technologies that should be evaluated.xxxix In contrast to the development of clinical guidelines, the Institute itself, in consultation with relevant stakeholders referred to as “consultees”, is responsible for the initial scoping of a given technology appraisal. This scope identifies the patient population for a given technology, the particular interventions being examined, what interventions these technologies will be compared with, and how “effectiveness” will be defined for the purpose of the appraisal. After the scope has been developed, NICE commissions another body – usually an academic unit – to undertake an evaluation on its behalf.xl This body, referred to as the assessment group, prepares an assessment report detailing the clinical and cost effectiveness of the technology in the manner defined in the scope. This initial report is released to consultees for xxxix The National Prescribing Centre provides this advice in relation to pharmaceuticals, while the National Horizon Scanning Centre provides advice on other health interventions. xl For example, the recent assessment of drug eluting stents for coronary disease (Appraisal number 71) was conducted by the Liverpool Reviews and Implementation Group at the University of Liverpool, while that on the removal of wisdom teeth was conducted by the NHS Centre for Reviews and Dissemination. 47 commentary, and then proceeds to an Appraisal Committee,xli who use this report and other evidence submitted to it by the consultees to determine the final appraisal of the technology. This phase consists of developing a draft appraisal that is distributed to consultees for commentary, followed by the production of the final appraisal incorporating, where appropriate, the views of the consultees. Consultees then have fifteen working days in which to appeal the results of the appraisal. The results of an appraisal can take the form of four possible recommendations. Routine use in all patients for whom the technology is appropriate. Restricted use of the technology amongst particular patient groups. This may be accompanied by a recommendation that further research on clinical and cost effectiveness be conducted. Use of the technology only within the context of clinical trials. That the technology should not be used. The Appraisal Committee also usually sets a point at which its recommendations are subject to review, although it has the capacity to define an assessment as “static” in which case any further review must specifically be requested by the Department of Health or Welsh National Assembly. xli This appraisal committee is appointed by NICE, and consists of health professionals, patient groups, health economists and health sector managers. The committee consists of both a core membership, and up to five additional co-opted members with expertise relevant to the technology being appraised. 48 1 Klein R. Dimensions of rationing: who should do what? British Medical Journal 1993, 307:309-311. Light DW, Hughes D. A sociological perspective on rationing: power, rhetoric and situated practices. In: Light DW, Hughes D, eds. Rationing: constructed realities and professional practices. Oxford: Blackwell Publishers, 2002:1-19. 3 World Health Organization. Report by the Secretariat, Round tables: lessons learned in world health, Fiftysecond World Health Assembly, A52/DIV/10. Manila: World Health Organization, 1999. 4 Ham C, Coulter A. International experience of rationing. In: Ham C, Robert G, eds. Reasonable Rationing: international experience of priority setting in health care. Maidenhead: Open University Press, 2003:4-15. 5 Tenbensel T. Health prioritisation as rationalist policy making: problems, prognoses and prospects. Policy and politics 2000; 28(3):425-40. 6 Ham C. Priority setting in health care: learning from international experience. Health policy 1997; 42(1):4966. 7 National Health Committee. The best of health 3: are we doing the right things and are we doing the right things right? Wellington: National Health Committee, 1997. 8 Council of Europe. Criteria for the management of waiting lists and waiting times in health care: report and recommendation No. R (99) 21 adopted by the Committee of Ministers of the Council of Europe on 30 September 1999. Strasbourg: Council of Europe, 2000. 9 Berg M, van der Grinten T. The Netherlands. In: Ham C, Robert G, eds. Reasonable Rationing: international experience of priority setting in health care. Maidenhead: Open University Press, 2003:115-40. 10 Ham C, Coulter A. International experience of rationing (or priority setting). In: Ham C, Coulter A, eds. The Global Challenge of Healthcare Rationing. Buckingham: Open University Press, 2000:1-12. 11 Rehnberg C. 1997. Sweden. In: Ham C, ed. Health Care Reform: learning from international experience. Buckingham: Open University Press, 1997:64-86. 12 Klein R, William A. Setting priorities: what is holding us back – inadequate information or inadequate institutions? In: Ham C, Coulter A, eds. The global challenge of healthcare rationing. Buckingham: Open University Press, 2000:15-26. 13 Ashon T, Cumming J, Devlin N. Prioritising health and disability support services: principles, processes and problems. A report to the National Health Committee on the HFA’s proposed prioritisation process. Wellington: National Health Committee, 1999. 14 Hauck K, Smith PC, Goddard M. The economics of priority setting for health care: a literature review. HNP discussion paper. Washington DC: The International Bank for Reconstruction and Development/ The World Bank, 2002. 15 Holm S. Goodbye to the simple solutions: the second phase of priority setting in health care. British Medical Journal 1998; 317:1000-2. 16 Arnesen T, Kapiriri L. Can the value choices in DALYs influence global priority-setting? Health policy 2004; 70:137-49. 17 Coast J. The Oregon plan: technical priority setting in the USA. In: Coast J, Donovan J, Frankel S, eds. Priority setting: the health care debate. Chichester: John Wiley & Sons, 1996:37-63. 18 Coast J. Efficiency: the economic contribution to priority setting. In Coast J, Donovan J, Frankel S, eds. Priority setting: the health care debate. Chichester: John Wiley & Sons, 1996:113-39. 19 Queen’s Centre for Health Services and Research Policy, The Joint Centre for Bioethics, The Change Foundation. Priority setting in Ontario’s hospitals: research report. Toronto: The Change Foundation, 2002. 20 Daniels N, Sabin J. The Ethics of Accountability in Managed Care Reform. Health affairs 1998;17(5):50-64. 21 Ham C, Robert G. Conclusions. In: Ham C, Robert G, eds. Reasonable Rationing: international experience of priority setting in health care. Maidenhead: Open University Press, 2003:141-56. 22 Norheim OF. Norway. In: Ham C, Robert G, eds. Reasonable Rationing: international experience of priority setting in health care. Maidenhead: Open University Press, 2003:94-114. 23 Oddsson K. Assessing attitudes towards prioritizing in healthcare in Iceland. Health policy 2003; 66(2):135146. 24 US Congress Office of Technology Assessment. Evaluation of the Oregon Medicaid Proposal. OTA-H-531. Washington, DC: Government Printing Office, 1992. 25 Ham C. Retracing the Oregon trail: the experience of rationing and the Oregon health plan. British Medical Journal 1998; 316:1965-1969. 26 Dunning AJ. Reconciling macro- and micro-concerns: objectives and priorities in health care. In: Organisation for Economic Co-operation and Development. Health care reform: the will to change. Paris: Organisation for Economic Co-operation and Development, 1996:59-65. 27 Ham C, Coulter A. 2000. Conclusions: where are we now? In: Ham C, Coulter A, eds. The global challenge of healthcare rationing. Buckingham: Open University Press, 2000:231-50. 2 49 28 Department of Health. Government response to the First Report from the Health Committee Session 19941995. Cm2826. London: Her Majesty’s Stationery Office, 1995. 29 Heritage J, Boyed E, Kleinman L. Subverting criteria: the role of precedent in decisions to finance surgery. In: Light DW, Hughes D, eds. Rationing: constructed realities and professional practices. Oxford: Blackwell Publishers, 2002:149-75. 30 McKinlay E, McLeod D, Dowell T, Howden-Chapman P. Clinical practice guidelines: a selective literature review. Wellington: New Zealand Guidelines Group, 2001. 31 National Health Committee. New technology assessment in New Zealand: discussion document. Wellington: National Health Committee, 2002. 32 House of Commons Select Committee on Health. Minutes of Evidence Taken Before the Health Committee Thursday 4th February 1999. http://www.parliament.the-stationery-office.co.uk/pa/cm199899/cmselect/cmhealth /222/9020401.htm 33 Robert G. The United Kingdom. In: Ham C, Robert G, eds. Reasonable Rationing: international experience of priority setting in health care. Maidenhead: Open University Press, 2003:64-93. 34 Maynard A, Bloor K, Freemantle N. Challenges for the National Institute of Clinical Excellence. British Medical Journal 2004; 329:229. 35 Halligan CJ. “Just what the doctor ordered”: Oregon’s Medicaid rationing process and public participation in risk regulation. The Georgetown Law Journal 1995; 83, 2697-725. 36 Wiseman V, Mooney G, Berry G, Tang KC. Involving the general public in priority setting: experiences from Australia. Social science and medicine 2003; 56, 1001-12. 37 Holm S. Developments in the Nordic countries – goodbye to the simple solutions. In: Ham C, Coulter A, eds. The global challenge of healthcare rationing. Buckingham: Open University Press, 2000:29-37. 38 Mullen P. Public involvement in health care priority setting: are the methods appropriate and valid? In: Ham C, Coulter A, eds. The global challenge of healthcare rationing. Buckingham: Open University Press, 2000:163-74. 39 Carson L, Cole-Edelstein L, Hardy M. Citizens’ juries in Australia: a discussion about protocols. 2000. http://activedemocracy.net/articles/protocol.pdf 40 Mooney GH, Blackwell SH. Whose health service is it anyway? Community values in healthcare. Medical journal of Australia 2004; 180:76-78. 41 Delap C. Citizens’ juries: reflections on the UK experience. PLA notes 2001; 40:39-42. 42 Florin D, Dixon J. Public involvement in health care. British medical journal 2004; 328:159-61. 43 Goold SD. Allocating health care: cost-utility analysis, informed democratic decision making, or the veil of ignorance? Journal of health politics, policy and law 1996; 21(1):69-98. 44 van Wersch A, Eccles M. Involvement of consumers in the development of evidence based clinical guidelines: practical experiences from the North of England evidence based guideline development programme. Quality in health care 2001; 10(1):10-16. 45 National Institute for Clinical Excellence. Guide to the technology appraisal process. Technology appraisals process series no. 1. National Institute for Clinical Excellence: London, 2001. 46 National Institute for Clinical Excellence. Information for the public and the NHS. The guideline development process series no. 1. National Institute for Clinical Excellence: London, 2001. 47 National Institute for Clinical Excellence. 2002. A Short Introduction to the Citizens Council. 2002. http://www.nice.org.uk/cat.asp?c=35528 48 Smith R. The triumph of NICE [Editor’s Choice]. British medical journal 2004; 329(7459): 0. 49 Gauld R. Beyond New Zealand’s dual health reforms. Social policy & administration 1999; 33(5): 567-85. 50 Ham C. Lessons and conclusions. In: Ham C, ed. Health care reform: learning from international experience. Buckingham: Open University Press, 1997:119-40. 51 Hornblow A. New Zealand’s health reforms: a clash of cultures. British medical journal 1997; 314:1892. 52 National Advisory Committee on Core Health and Disability Support Services. Core health and disability support services for 1993/1994: first report of the National Advisory Committee on Core Health and Disability Support Services. Wellington: National Advisory Committee on Core Health and Disability Support Services, 1992. 53 Tenbensel T. Does more evidence lead to better policy? The implications of explicit priority-setting in New Zealand’s health policy for evidence-based policy. Revision of paper presented at the 2003 Conference of the New Zealand Political Studies Association, University of Auckland, April 12-14, 2003. 54 Edgar W. Rationing – how the public has a say. In: Coulter A and Ham C, eds. The global challenge of healthcare rationing. Buckingham: Open University Press, 2003; 175-191. 55 Upton S. Your health and the public health: a statement of government health policy. Wellington: Minister of Health, 1991. 50 56 National Advisory Committee on Core Health and Disability Support Services. The best of health 3: are we doing the right things and are we doing the right things right? Wellington: National Advisory Committee on Core Health and Disability Support Services, 1997. 57 Bloomfield A. New Zealand. In: Ham C, Robert G, eds. Reasonable Rationing: international experience of priority setting in health care. Maidenhead: Open University Press, 2003:16-41. 58 Pharmac. Operating policies and procedures of the Pharmaceutical Management Agency (PHARMAC). Wellington: Pharmac, 2001. 59 Pharmac. Advice to incoming Minister of Health, background paper: Pharmac and pharmaceutical issue. Wellington: Pharmac, 2002. 60 Ministry of Health. Current prioritisation processes in New Zealand. Wellington: Ministry of Health, 2003. 61 New Zealand Guidelines Group. Mission and Values. 2004. http://www.nzgg.org.nz/index.cfm?fuseaction=about&fusesubaction=article&documentid=6&articleID=1 62 The New Zealand Guidelines Group. The local adaptation of three mental health guidelines: assessment of processes for local adaptation and implementation. Wellington: New Zealand Guidelines Group, 2003. 63 Derrett S, Devlin N, Hansen P, Herbison P. Prioritizing patients for elective surgery: a prospective study of clinical priority access criteria in New Zealand. International journal of technology assessment in health care 2003; 19(1): 91-105. 64 Roake J. Managing waiting lists. In Gauld R, ed. Continuity amid chaos: health care management and delivery in New Zealand. Dunedin: University of Otago Press, 2003:107-121. 65 Derrett S, Paul C, Herbison P, Williams H. Evaluation of explicit prioritisation for elective surgery: a prospective study. Journal of health services research and policy 2002; 7(suppl 1):14-22. 66 Health Funding Authority. Priority setting and resource allocation. Paper prepared for the Health Funding Authority Board. Wellington: Health and Disability Analysis Unit, Midland Division of the Health Funding Authority, 1998. 67 Ministry of Health. Final Service Coverage Schedule 2003/04. Wellington: Ministry of Health, 2003. 68 Ministry of Health. Operational Policy Framework. Wellington: Ministry of Health; 2003. 51