Biology B-3-1-1
advertisement

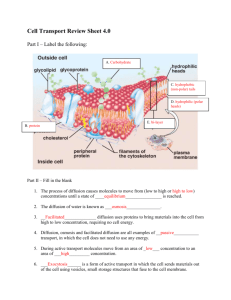
Lesson Code (Course - Master Objective # - Benchmark # - Lesson # - #) Biology B-3-1-1-1 Title - Author Cell Transport – Rhonda Cook Benchmark/Expectation/Concept/Process/Skill 3-1: Compare and contrast ways in which cells transport materials in and out of the cell. Relevant Goals Show-Me Process 1.6 Show-Me Science Content 3 Missouri Science G(C)LE(s) 3.2.A: c 3.2.F: a-c SJSD 6 National (5-8, 9-12) C Learning Path Previous Learning 3.2.A: a. Describe how the cell membrane helps regulate the transfer of materials in and out of the cell 3.2.B: c. Describe the importance of the transport and exchange of oxygen and carbon dioxide to the survival of the organism 3.2.C: e. Identify the importance of the transport and exchange of nutrient and waste molecules to the survival of the cell and organism 3.2.F: a. Predict the response the body may take to maintain internal balance during an environmental change (e.g., shivering when cold, slowing metabolism when food supply decreases or when dehydrated, adrenaline rush when frightened) Targeted Learning 3.2.A: c. Explain physical and chemical interactions that occur between organelles (e.g. nucleus, cell membrane, chloroplast, mitochondrion, ribosome) as they carry out life processes. 3.2.F: a. Explain the significance of the selectively permeable membrane to the transport of molecules 3.2.F: b. Predict the movement of molecules across a selectively permeable membrane (i.e., diffusion, osmosis, active transport) needed for a cell to maintain homeostasis given concentration gradients and different sizes of molecules 3.2.F: c. Explain how water is important to cells (e.g., is a buffer for body temperature, provides soluble environment for chemical reactions, serves as a reactant in chemical reactions, provides Future Learning hydration that maintains cell turgidity, maintains protein shape) Possible Misconceptions Teacher Notes Engage: Engages the learner’s mind in the concept, process, or skill to be learned, and makes connections between prior experiences and the current learning goals. Ask the following questions to generate discussion to get the students thinking. What is transportation? What is its purpose? What are some different types of transportation? (make sure that motor vehicles, nonmotored vehicles and body power are all considered) When considering cells and cell structure, why would it be important for molecules to be able to move around? Set up the activity “Spread the Smell”. This experiment is about diffusion. Explain that diffusion happens in liquids as well but it takes a bit longer for diffusion to occur in liquids. Materials you will need: Jar of coffee Bottle of vinegar Bottle of spray perfume An orange Room freshener (Aerosol spray) Basil Plant (herb) (Choose at least two of the above) A fan (optional) Some helpers/volunteers Some blind folds (optional) A stop clock (optional) A note pad and pencil Spread the Smell Steps: 1. Place your items of on a table at the front of the room. If you are going to use a fan place it at the front of the room with you so that the air blows towards you participants. 2. Have your helpers form a line at the front of the room. Then ask each volunteer to (excluding the first person in the line) take one step backwards to form a bit of a gap between each of them. 3. Ask your helpers to place their blind fold on or ask them to keep their eyes closed. 4. Leave the fan switched off. Ask your helpers to raise their hand if/when they smell something different in the air. 5. Open the lid or spray one of your items and see how long it takes for the aroma to reach each of your helpers. You could record your results by using a stop clock to see how long it takes for each smell to reach each person. One test could be with the fan off and the other could be with the fan switched on. If you choose to use a basil plant in your experiment it is probably best if you hold the plant and walk past each person in the line. As you get close to each person place one hand on the top leaves of the plant and just fan them slightly with your hand to help the aroma to move through the air. It is amazing how powerful a basil plant can smell. This experiment is about diffusion. What is diffusion? Diffusion happens when particles mix with each other without anything moving them. Smells move around a room and some end up in our nose where we have smell detectors (our sense of smell). Gas particles move on their own in the air and therefore the smells mix and spread. With the use of a fan the smells move through the air faster. Diffusion happens in liquids as well but it takes a bit longer for diffusion to occur in liquids. Formative Assessment(s): Explore: Provides or creates a common experience for all learners and helps the teacher identify the prior knowledge of each learning to build on. This stage allows time for the learner to explore their ideas. This stage should be concrete and meaningful to the learner. Discuss the following terms and definitions with the class. Passive Transport Movement of molecules from a more crowded to a less crowded area WITHOUT the use of energy. Movement occurs when there are unequal concentrations of a substance inside and outside the cell. Diffusion Diffusion is the movement of molecules from an area of higher concentration to a region of lower concentration. Osmosis Diffusion of water through a membrane Active Transport Movement of molecules from a more crowded to a less crowded area WITH the use of energy. Molecules are “carried” into or out of the cell using some the cell’s energy. Homeostasis The ability or tendency of an organism or cell to maintain internal equilibrium by adjusting its physiological processes. Selective Permeability Selective permeability means that the cell membrane has some control over what can cross it, so that only certain molecules either enter or leave the cell. (Like a screen on a window that will let air pass through, but not many insects.) Notes on Diffusion and Osmosis. These can be printed and used as handouts. There are also a couple of animated examples of diffusion and osmosis. http://www.biologycorner.com/bio1/diffusion.html# Notes on Active Transport. Can also be used as a handout. http://www.biologycorner.com/bio1/active.html Osmosis in Potatoes Experiment The following experiment is a fun and easy way to see the effects of plant osmosis on a plant by comparing two different potatoes placed in different types of water These are the materials needed to view osmosis in action: - 2 Potatoes - 2 Plates - Salt - Water - Knife Methods: Fill both of the dishes with water and add about two tablespoons of salt to one of the dishes. Using the knife, cut the potato in half lengthwise. Then Place each piece flat side down in to one of the plates of water. Now simply let the two potato pieces soak in the water for a few hours. After this time has passed flip each potato over and look for differences. When looking at the potato pieces you can clearly see a difference between the two. Let’s take a closer look at each of the potato pieces! The potato slice that has been soaking in freshwater. Not much of a difference here, only that the potato is a little more rigid then before. This is because there is the more salt and other dissolved chemicals within the potato then the surrounding water. This means that the water will move into the potato. This potato slice that has been soaking in saltwater. This potato pieces looks substantially different from the original and the other slice. It seems to have wilted, gotten very soft and flexible. Why did that happen? It has to do with a process called osmosis. The potato is made up of tiny, living units called cells. Each cell is surrounded by a cell membrane which acts much as your skin does. It keeps the cells parts inside and keeps other things outside, protecting the cell. While this membrane stops most things, water can pass through it. The water tends to move towards higher concentrations of dissolved chemicals. That means that if the water outside the cell is saltier than the water inside, water will move from the inside of the cell to the outside. As the water left the cell it was much like letting the air out of a balloon. As more and more of the cells lost water, the slice of potato became soft and flexible. Formative Assessment(s): Explain: Allows the learner to construct an explanation (claim evidence reasoning). The teacher provides information in common terms to increase the accuracy of the explanation. Diffusion Lab http://www.biologycorner.com/worksheets/diffusionlab.htm Diffusion Lab Introduction: In this lab you will observe the diffusion of a substance across a semi permeable membrane. Iodine is a known indicator for starch. An indicator is a substance that chances color in the presence of the substance it indicates. Watch as your teacher demonstrates how iodine changes in the presence of starch. Prelab Observations: Describe what happened when iodine came into contact with starch. Procedure: 1. Fill a plastic baggie with a teaspoon of corn starch and a half a cup of water tie bag. (This may already have been done for you) 2. Fill a beaker halfway with water and add ten drops of iodine. 3. Place the baggie in the cup so that the cornstarch mixture is submerged in the iodine water mixture. 4. Wait fifteen minutes and record your observations in the data table. 5. While you are waiting, answer the questions. Questions: 1. Define diffusion. 2. Define osmosis 3. What is the main difference between osmosis and diffusion 4. Why is iodine called an indicator? 5. Molecules tend to move from areas of _______ concentration to areas of ______ concentration. What is in the Bag? We’re going to think about concentrations now, which substances are more or less concentrated depends on which one has the most stuff in it. 1. Is the baggie or beaker more concentrated in starch? 2. Is the baggie or beaker more concentrated in iodine? 3. Iodine solution: is the baggie or the beaker hypertonic? 4. Starch solution: is the baggie or the beaker hypertonic? 5. Which one is hypotonic in relation to starch, baggie or beaker? Make Some Predictions 1. If the baggie was permeable to starch, which way would the starch move, into the bag or out of the bag? ________ 2. If the baggie was permeable to iodine, which way would the iodine move, into or out of the bag? _______ 3. If the baggie was permeable to iodine, what color would you expect the solution in the baggie to turn? _______ What about the solution in the beaker? ___________ 4. If the baggie was permeable to starch, what color would you expect the solution in the baggie to turn? ________ What about the solution in the beaker? _________ 5. Make a prediction about what you think will happen: Data Table Starting Color Color after 15 minutes Solution in Beaker Solution in Bag Post Lab Analysis 1. Based on your observations, which substance moved, the iodine or the starch? 2. How did you determine this? 3. The plastic baggie was permeable to which substance? 4. Is the plastic baggie selectively permeable? 5. Sketch the cup and baggie in the space below. Use arrows to illustrate how diffusion occurred in this lab. 6. What would happen if you did an experiment in which the iodine solution was placed in the baggie, and the starch solution was in the beaker? Be detailed in your description. 7. Why is it not a good idea to store iodine in a plastic bag? Formative Assessment(s): Have the students use the Claim-Evidence-Reasoning model to explain this experiment. Have them develop a testable question. The claim is the answer to that question. Elaborate: Pushes learner understanding, building on current understanding to increase the depth and breadth of understanding. Allow the learner to extend and apply the concepts, processes, or skills. Allows learner to experience new situations to apply to their learning. Additional Labs 1. HONEY, I SHRUNK THE CARROTS - Carrot Osmosis Activity Background: Learner should know the main parts of a cell. Learner should understand particle movement. Summary: Students will use carrots as cell models and demonstrate diffusion and osmosis. Students will measure and observe the changes in the mass and size of the carrot. Materials: Two 400 mL beakers string measuring tape or meter stick salt distilled water triple beam balance carrots Safety concerns: Teachers and students, be sure to keep all Glass, Chemical, and Sharp instrument Safety Rules that are specified by the teacher and in all general laboratory experiences. Student Procedures: Carrot Experiment 1. 2. 3. 4. Fill two beakers with equal amounts of water. Add 15 g salt to one beaker and label it "Salt Water". Cut a carrot in half. Tightly tie a piece of string two cm below the cut end of both pieces. Place one carrot half (cut end down) in the "Salt Water" beaker. Place the other carrot with cut end down in the "Fresh Water" beaker. Allow carrots to remain undisturbed for 24 hours. Students form a hypothesis. Remove carrots and observe them and the tightness of the strings. Record data. 5. At the conclusion of your experiment, answer the following questions (realize that some require deeper thinking than others? Did the thread become loose in fresh water or salt water? Did the thread become tight thread in fresh water or salt water? Did the carrot develop a soft texture in fresh water or salt water? Did the carrot develop a firm texture in fresh water or salt water? In which type of water did the carrot cells increase in cell size (freshwater or salt water?) In which type of water did the carrot cells decrease in cell size (freshwater or salt water?) Was there a loss of water by cells in fresh water or salt water? Did the cells gain of water in either fresh water or salt water? Deeper thinking questions What was the purpose of having you tie thread on each carrot? In which kind of water did the carrot cells lose water? o What evidence supports your conclusion? In which kind of water did the carrot cells gain water? o What evidence did you use to determine this? What do you think would happen to human blood cells if they were placed in a beaker of salt water? 2. CELL AND DIFFUSION LAB Background: Learner should know the main parts of a cell. Learner should understand particle movement. Summary: Students will use bags as cell models and demonstrate diffusion. Observations: Have students observe the properties of iodine solution. (liquid, tan colored) Have students observe what happens to the color of iodine as it comes in contact with starch in the form of bread. (The iodine turns black in the presence of starch it is an indicator of starch. How might a bag be like a cell? (It can hold things like a cell. The bag would be the cell membrane.) Materials: For use by the class: Box of cornstarch and iodine solution For each group of students: small plastic bags, small rubber, bands, 1 - cup measuring cup, scissors, 600 mL beaker, access to warm and lukewarm water Safety concerns: Teachers and students, be sure to keep all Glass, Chemical, and Sharp instrument Safety Rules that are specified by the teacher and in all general laboratory experiences. Student Procedures: 1. Add one teaspoon of cornstarch to 1 cup of water. Stir until the cornstarch is dissolved. 2. Pour the mixture into a small plastic bag. Wrap a rubber band around the top of the bag to seal the bag. 3. Pour 100 mL of warm water into a 600 mL beaker. 4. Carefully place the bag with the cornstarch water into the 600 mL beaker. 5. Let it set for a few minutes and observe what happens. 6. Answer the following questions: Why did the water inside the bag turn black? What part of the cell is the bag like? Which process occurred, diffusion or osmosis? Did the starch move out of the bag? How do you know? Extension: Are there any other indicators that could be used to show the diffusion of particles across the bag wall? Does the temperature of the water affect how long it takes for the iodine to move into the cell? Formative Assessment(s): Evaluate: Provides and opportunity for the learner to assess their own understanding and be able to demonstrate the depth and breadth of that understanding to others. Teacher assesses the learner’s level of understanding and mastery. Summative Assessment(s): The student will design a poster using one of the labs above that they have already completed. The poster will need to show the process of osmosis or diffusion as it occurred in the experiment. Print out Osmosis or Diffusion Poster Scoring Guide. Additional Resources: Text: Science Explorer: Cells and Heredity, pp. 32-37 Websites: http://www.biologycorner.com/bio1/diffusion.html#, http://www.biologycorner.com/bio1/active.html, http://www.biologycorner.com/worksheets/diffusionlab.htm, Video: Attachments: Teacher Review: Include date, course, and name of teacher.