Name: A&P LAB: Membrane Transport Objectives: Explain the role
advertisement

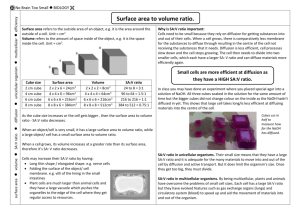
Name:_____________________________________________ A&P LAB: Membrane Transport Objectives: Explain the role of the cell membrane in passive transport. Understand and explain the terms diffusion, osmosis, selectively permeable membrane, and equilibrium. Predict and observe the effects mass and concentration on rate of osmosis and diffusion. Observe and explain the problems cells face if they become too large. Background: The absorption of nutrients, excretion of cellular wastes, and the exchange of respiratory gasses are life processes which depend upon the efficient transport of substances into, out of, and throughout living cells. The process of diffusion can be easily visualized by adding Lugol’s iodine solution to a glass of water. Initially, the iodine remains in a small area in the water, dying it a yellowish. Over time, the molecules of iodine collide with each other, and with molecules of water, and the iodine eventually disperses throughout the entire glass of water, resulting in a golden brown color in the water. Much like the iodine diffuses through the glass of water, many important substances move into and out of cells by diffusion. Diffusion is the movement of a substance through a concentration gradient from high to low concentration. It is an example of passive transport because it requires no energy on the part of the cell. For this reason, diffusion is one of the most common and efficient means by which substances are transported between cells and their environment. The cell membrane is the selectively permeable barrier whose total surface area is important in regulating the substances that diffuse into or out of the cell. In order to represent the diffusion of materials into the cell, cubes of potato can be used. Iodine reacts with the starch in the potato to turn a blue-black color. This provides a clear visual as to how far the iodine diffuses into the potato in a given period of time. The distance can be measured and used to calculate rate of diffusion (Rate = distance/time). Particles such as iodine are not the only things that diffuse to maintain a state of equilibrium. Water will also diffuse through osmosis to maintain equilibrium. Since not all particles are able to cross the semi-permeable membrane, it is important to maintain a relatively equal concentration of water in the intracellular and extracellular environments. If there is too much water inside the cell compared to the surrounding environment, water will leave the cell and the cell will shrivel, substantially disrupting cellular function. If there is too much water outside the cell, water will enter the cell and it may burst. Methods: PART I – Diffusion & Cell Size 1. 2. 3. 4. Obtain one beaker and one large and one small potato cube. Place the potato cubes into the beaker and fill it with just enough water to cover the large cube. Place enough iodine into the beaker to turn it dark yellow or brownish. Put the beaker aside for 45 minutes to an hour and complete Part II. PART II – Osmosis 1. 2. 3. 4. 5. 6. 7. 8. Obtain 4 beakers and label them 0%, 1%, 3% & 5% NaCl Prepare 100mL of each of the solutions for the appropriate beaker. Obtain 4 potato cyclinders. Rinse them quickly in tap water and dry them with a paper towel. Weigh each one and create a data table to record the initial weights of each cylinder as well as three more weights after 15, 30 and 45 minutes. Place each cylinder in one of the beakers of solution. (NOTE: Make sure to keep track of which cylinder goes in which beaker.) After 15 minutes, remove the cylinders from the beakers. Dry them off quickly, weigh each one and put it back into the cylinder. (AGAIN be sure to keep track of which cylinder came from which beaker and put it back into the correct beaker. Repeat step 6 after 30 minutes and 45 minutes. Since each potato cylinder had a different starting mass, create a second data table to record the % weight change after 15, 30 and 45 minutes. % weight change = (weight at time “x” – initial weight/initial weight) x 100 LAB REPORT: Results: PART I Fig 1: Scaled diagram of Cube 1 (small cube) – shade and label the distance of diffusion Fig 2: Scaled diagram of Cube 2 (large cube) - shade and label the distance of diffusion PART II Table 1: Weight (g) of potato cylinders over 15 minute Intervals Table 2: Percent Weight change of potato cylinders over 15 minute intervals Analysis: PART I - Below the sketches calculate the % diffusion into each cube - Did the cube size have an effect on the rate of diffusion? - Calculate the approximate surface area to volume ratio for each cube. o - SA = L x W x #sides V = L x W x H What happens to the SA:V ratio as cube size increases? Relate this to the need for cell division to maintain many small cells rather than a higher number of large cells. PART II - Did any of the cylinders gain weight? If so, what made them gain weight? - Did any of the cylinders lose weight? If so, how did that occur? - Refer to your data to approximate the saline concentration of the potato cells. - Burn victims are often wrapped in cloth that has been soaked in normal saline solution, which is a 0.9% salt solution. A standard IV drip for hydration purposes at the hospital is also a normal saline solution as is lactated Ringer's solution, often used for fluid resuscitation after a blood loss due to trauma, surgery, or a burn injury. Refer to your lab data to explain why this precise concentration of 0.9% is so important. - Graph the data as Figure 1: % Weight Change v. Time (min) o There will be a line for each cylinder o Follow graphing guidelines from Organism and pH (buffer) Lab (This is posted in A&P files) Conclusion: Use the following prompts to develop your conclusion paragraph. The conclusion should be composed of welldeveloped, complete sentences. Refer to data for support and clarification when appropriate. Address the objectives and summarize what you learned as well as what the results indicate. Do the results support or refute your hypothesis (pre-lab predictions) or were your results inconclusive? Were there any sources of error/problems with the experimental design? If so, how could you address these issues? What new questions have you developed based on the results of this experiment that could lead to further investigation? Name:______________________________________________ Pre-Lab Discussion/Hypotheses Directions: Respond to the following questions in complete sentences. This will be handed in stapled to your graph and calculations and will be counted as part of your lab grade. PART I: 1. What types of molecules are in the potato? What types of molecules are found in the beaker? 2. Of the molecules listed above, which do you think could cross a semi-permeable membrane? (Think about size/structure of molecules as discussed Chapter 2.) 3. Based on your answers above, make a prediction about what you expect the results to be. PART II: 1. Distinguish between hypotonic, isotonic and hypertonic solutions. 2. Make a prediction about what will happen to a cell (potato) placed each type of solution.