measuring the rate of osmosis using “deshelled” chicken eggs
advertisement

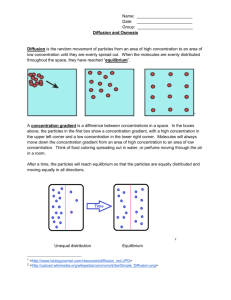
MEASURING THE RATE OF OSMOSIS USING “DESHELLED” CHICKEN EGGS (Effect of Solute Concentration Upon Rate/Degree of Osmosis in Chicken Eggs). Introduction If a cell is to perform its functions, it must maintain a steady state in the midst of an everchanging environment. This constancy is maintained by the regulation of movement of materials into and out of the shell. To achieve this control, cells are bounded by a delicate membrane that differentiates between different substances, slowing down the movement of some while allowing others to pass through. Since not all substances penetrate the membrane equally well, the membrane is said to be differentially permeable. The external and internal environment of cells is an aqueous solution of dissolved inorganic and organic molecules. Movement of these molecules, both in the solution and through the cell membrane, involves a physical process called diffusion – a spontaneous process by which molecules move from a region in which they are highly concentrated to a region in which their concentration is lower. A special kind of diffusion is the phenomenon of osmosis. Simply defined in biological systems, osmosis is the diffusion of water through a differentially permeable membrane from a region in which it is highly concentrated to a region in which its concentration is lower. More often, however, osmosis is defined in terms of the effects that solutes have on the thermodynamic activity of water (i.e., the activity of the water molecule due to the kinetic energy of motion). For example, the addition of a solute to water tends to decrease the activity of the water. In other words, as more water molecules are displaced by solute molecules, the activity of the water goes down. Thus, in thermodynamic terms, water diffuses across membranes from a region in which the thermodynamic activity of water is high (low solute concentration) to one in which the thermodynamic activity is low (high solute concentration). We use the terms hypotonic, hypertonic and isotonic in referring to the relative concentrations of solute particles of different solutions. Below are the definitions of the three terms according to Curtis, 1983. Hypotonic (Gk. hypo, under + tonos, tension): Of two solutions of different concentration, the solution that contains the lower concentration of solute particles; water moves across a semi-permeable membrane into a hypertonic solution. Hypertonic (Gk. hyper, above + tonos, tension): Of two solutions of different concentration, the solution that contains the higher concentration of solute particles; water moves across a semi-permeable membrane into a hypertonic solution. Isotonic (Gk. isos, equal + tonos, tension): Having the same concentration of solutes as another solution. If two isotonic solutions are separated by a semi-permeable membrane, there will be no net flow of water across the membrane. It should be noted that the number of solute particles is the thing that affects the relative activity of the water, not the kind of particles. Heat increases the motion of molecules. Therefore, we expect an increase in temperature to speed up the rate of osmosis, regardless of which direction the solvent is moving. This is diagrammatically shown below. A. Water enters cell B. Water leaves cell 0.1M solute 0.9M solute particles particles (i.e., sucrose) (i.e., sucrose) 0.5M solute particles (i.e., proteins) 0.5M solute particles (i.e., proteins) H2O H2O (H2O activity lower than outside) (H2O activity higher than outside) (H2O activity (H2O higher than lower inside cell) inside activity than cell) HYPOTONIC HYPERTONIC THE EXPERIMENT Part I: Effect of Solute Concentration Each group of students will be given 6 chicken eggs from which the shell has been dissolved away. The remaining membrane (the shell membrane) is differentially permeable. We will assume that each egg has approximately the same concentration of solute in this membrane, and based on the rate of osmosis, will attempt to determine what this concentration must be. Weight each egg separately to the nearest 0.1g and record the weights in Table 1 at time “0”. Place eggs 1, 2, 3, 4, 5, and 6 into separate beakers containing solutions of distilled water, 10% sucrose, 20% sucrose, 30% sucrose, 40% sucrose and unknown sucrose solution respectively. At 15 minute intervals, that is after 15, 30, 45, 60, and 75 minutes, remove the eggs from the beakers; carefully wipe off all excess water; and again weigh each egg separately. Record the weight in Table 1. Plot the changes in weight of each of the eggs against time on a piece of graph paper. Which solutions would you say were hypotonic to that of the eggs? Which of them were hypertonic? Isotonic? What would you expect to happen if an egg was put into a sixth beaker containing a 50% sucrose solution? Table I: Weight Change of Eggs (g) vs Time (minutes) Time (Min.) 0% suc 10% suc 20% suc 30% suc 40% suc Unknown 40% suc Unknown 0 15 30 45 60 75 Table II: Weight Change of Eggs (g) vs Time (minutes) Time (Min.) 0 15 30 45 60 75 0% suc 0 10% suc 0 20% suc 0 30% suc 0 0 0 Table III: Total Weight Change of Eggs (g) vs Sucrose Concentration (%) Sucrose Conc. 0%Suc Tot. Wt. Change 10%Suc 20%Suc 30%Suc 40%Suc In order to fill out table 3, extract data on the 75th minute row of table 2 and place them on table 3. Lab Report for Movement of Materials Across Cell Membranes To assist you in your understanding of this laboratory, you are asked to prepare the following graphs using Microsoft excel and answer the questions at the end of each section. 1. Prepare a graph in which you plot the weight change of the six (6) eggs (use data from table 2), which have been placed in solutions of varying solute (sucrose) concentrations, as a function of time. You will have six (6) lines on this graph (one for each egg). This graph indicates the weight change of eggs (g) vs time (minutes) (+) Weight Change of Eggs (grams) (-) 0 0 15 30 45 60 75 a. What conclusion can you draw from the data in this graph? b. Which solutions were hypotonic? Hypertonic? Isotonic? 2. Prepare a graph in which you plot total weight change of the eggs (g) placed in varying solute (sucrose) solutions against sucrose concentration (%). You, in effect, will be constructing a type of standard curve. Use the data in table 3 to construct this graph. (+) Total Weight Change (grams) 0 (-) 0 10 20 30 40 Sucrose Concentration (%) Using this graph, you should be able to: 1. Determine the isotonic point of the contents of a chicken egg. (Hint: where your curve crosses the 0 line, read down to the concentration axis and record the value you obtain.) 2. Determine the concentration of the unknown solution. (Hint: locate on the total weight change axis, the value representing the total weight change of the egg placed in the unknown solution. Read across to your standard curve and then down to the concentration axis and record the value you obtain.)