NOTES MOVEMENT IN & OUT OF CELLS.doc
advertisement

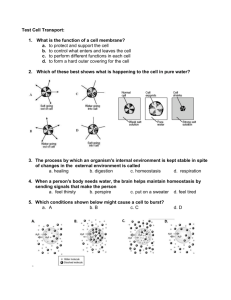
IGCSE Biology 3 Movement in & out of cells To get into or out of cells, dissolved substances have to cross the cell membranes. solute ~ particles in solution ( e.g. glucose, sodium ions, chloride ions ). solvent ~ liquid in which the particles are dissolved ( e.g. water ). Solute and solvent molecules move around randomly. Solutes can move into and out of cells by diffusion and osmosis Define diffusion as the net movement of molecules from a region of their higher concentration to a region of their lower concentration down a concentration gradient, as a result of their random movement. Diffusion Dissolved substances have to pass through the cell membrane to get into or out of a cell. Diffusion is one of the processes that allow this to happen. Diffusion occurs when particles move from a region where they are in high concentration to a region where they are in low concentration. Diffusion happens when the particles are free to move. This is true in gases and for particles dissolved in solutions. Particles diffuse down a concentration gradient, from an area of high concentration to an area of low concentration. The greater the difference in concentration, the faster the rate of diffusion. Describe the importance of diffusion of gases and solutes and of water as a solvent. Examples of diffusion: location particles move from to intestine digested food products intestinal cavity blood in capillary of villus lungs oxygen alveolar air space blood circulating around the lungs In the intestine, the concentration of molecules used by the body for energy, growth and repair are in higher concentration in the intestinal cavity than in the blood. In the lungs, the blood will continue to take in oxygen from the alveolar air spaces provided the concentration of oxygen there is greater than in the blood. Oxygen diffuses across the alveolar walls into the blood, and the circulation takes the oxygen-rich blood away. All known forms of life depend on water. Water is vital both as a solvent in which many of the body's solutes dissolve and as an essential part of many metabolic processes within the body. Water is a good solvent. Water serves to suspend the red blood cells to carry oxygen to the cells. It is the solvent for the electrolytes and nutrients needed by the cells, and also the solvent to carry waste material away from the cells. Define osmosis as the diffusion of water molecules from a region of their higher concentration (dilute solution) to a region of their lower concentration (concentrated solution), through a partially permeable membrane. Dissolved substances pass into and out of cells by diffusion. Water passes into and out of cells by osmosis. Osmosis is the movement of water from a less concentrated solution to a more concentrated solution through a partially permeable membrane. Water can move across cell membranes because of osmosis. For osmosis to happen you need: two solutions with different concentrations a partially permeable membrane to separate them A partially permeable membrane will let some substances pass through it, but not others. In osmosis it is only water that moves in the following direction: from a region of high water concentration to a region of low water concentration. from a more dilute solution ( with a low solute concentration ) to a more concentrated one ( with a high solute concentration ). from a weaker solution to a stronger one. Describe the importance of osmosis in the uptake of water by plants, and its effects on plant and animal tissues. Animal cells need a constant supply of food & oxygen. Food & oxygen dissolved in the blood moves from the capillaries into cells by diffusion. As cells use food & energy, they produce waste in the form of carbon dioxide. Carbon dioxide diffuses from cells into the blood. Osmosis is important to plants. They gain water by osmosis through their roots. Water moves into plant cells by osmosis, making them turgid so they that able to hold the plant upright. Osmosis in animal cells: If animal cells are placed in a solution that has a higher solute concentration than the cytoplasm, then water will leave the cell by osmosis, until it shrinks and dies. If animal cells are placed in a solution that has concentration than the cytoplasm, then water cell by osmosis until it bursts. cytoplasm. a lower solute will enter the This is why it is vital that we maintain the concentration of our body fluids at an equal solute concentration to our cells’ Describe & explain the importance of a water potential gradient in the uptake of water by plants. Osmosis in plant cells: If plant cells are placed in a solution that has a higher solute concentration than the cytoplasm, then water will leave the cell by osmosis, and the cell membrane separates from the cell wall (plasmolysis). This will cause a plant to wilt. If plant cells are placed in a solution that has a lower solute concentration than the cytoplasm, then water will enter the cell by osmosis until it is fully turgid, and the cell wall prevents any more water entering. This is important in enabling plants to remain upright.