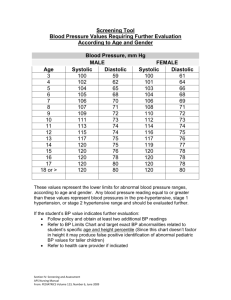
Heart Failure with Preserved Ejection Fraction
advertisement

Heart Failure with Preserved Ejection Fraction By Sheryl L. Chow, Pharm.D., FCCP, BCPS (AQ Cardiology) Reviewed by Barry E. Bleske, Pharm.D., FCCP; Timothy Murray, Pharm.D., BCPS; and Mary H. Parker, Pharm.D., BCPS (AQ Cardiology) Learning Objectives (A) or eject blood. Heart failure has a high incidence, with an estimated 550,000 new cases diagnosed annually in the United States, and high mortality, with one-half of these patients likely to die within 5 years. Substantial increases in the incidence and costs associated with this disease have occurred; its sequelae: in 2010, $40 billion was spent on health care related to HF, with about 300,000 deaths in the United States (Roger 2011). A large proportion of all patients with HF are now defined as having heart failure with preserved ejection fraction (HFpEF), also referred to as preserved HF, diastolic HF, or HF with preserved systolic function. These patients will generally show classic signs and symptoms of systolic HF in the presence of what is considered normal ejection fraction (EF) (i.e., 40%–65%) (Smith 2003; Gaasch 1994). Despite the substantial risk of morbidity and mortality, effective treatment options are largely empiric, given the lack of evidence to date. Current guidelines focus on treating comorbidities and improving signs and symptoms of patients with HFpEF. Additional large randomized controlled studies are needed in this patient population to improve our current understanding of the mechanisms, pathophysiology, and effective treatment strategies to improve clinical outcomes. 1. Demonstrate the association between heart failure with preserved ejection fraction (HFpEF) and survival. 2. Given a patient with heart failure (HF), recognize HFpEF on the basis of clinical signs and symptoms, physical examination, echocardiography, and radiographic findings. 3. Classify patients at high risk of hospitalization and mortality through assessing risk factors, clinical presentation, and interpretation of biomarkers. 4. Distinguish the clinical presentation, diagnosis, and treatment strategies of HFpEF from those of HF with reduced ejection fraction. 5. Given a patient with HFpEF, develop an individualized treatment plan based on current evidence. 6. Assess the potential role of future pharmacotherapies for HFpEF. Introduction (A) Heart failure (HF) is a complex clinical syndrome that can result from any cardiac structural or functional disorder that impairs the ability of the ventricle to fill with Baseline Knowledge Statements Readers of this chapter are presumed to be familiar with the following: ■■ Criteria for NYHA functional classification in patients with heart failure ■■ Pharmacologic management of patients with systolic heart failure as recommended by the American College of Cardiology/ American Heart Association and Heart Failure Society of America guidelines Additional Readings The following free resources are available for readers wishing additional background information on this topic. ■■ American College of Cardiology/American Heart Association Practice Guideline: 2009 Focused Update Incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. Circulation 2009;119:e391-e479. ■■ Heart Failure Society of America. 2010 Comprehensive Heart Failure Practice Guideline. ■■ Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. ESC Guidelines for the Diagnosis of Acute and Chronic Heart Failure 2012. Eur Heart J 2012. PSAP 2013 • Cardiology/Endocrinology 1 Heart Failure with Preserved Ejection Fraction (HFrEF), an increase in preload or left ventricular end diastolic pressure is produced as a result of a reduced contractility, EF, and ventricular compliance. In contrast, HFpEF is associated with an increase in left ventricular diastolic pressure through abnormalities of diastolic function, specifically related to impaired relaxation and stiffness. Although both types of HF eventually produce elevated filling pressures, the mechanisms associated with each are quite distinct. Table 1-1 describes the various etiologies of HF; Table 1-2 compares characteristics of HFpEF and HFrEF. Abbreviations in This Chapter (A) ACE Angiotensin-converting enzyme ARB Angiotensin receptor blocker CHARM- Candesartan in Heart Failure: Preserved Assessment of Reduction in Mortality and Morbidity–Preserved study EF Ejection fraction HF Heart failure HFpEF Heart failure with preserved ejection fraction HFrEF Heart failure with reduced ejection fraction I-PRESERVE Irbesartan in Heart Failure with Preserved Ejection Fraction LVEDP Left ventricular end-diastolic pressure LVEF Left ventricular ejection fraction NT-proBNP N-terminal pro–B-type natriuretic peptide NYHA New York Heart Association RAAS Renin-angiotensin-aldosterone system Impaired Relaxation (C) Impaired cardiomyocyte relaxation contributes to diastolic dysfunction, which has been associated with elevated filling pressures. The degree of myocardial relaxation can affect hemodynamic parameters by increasing pulmonary vein pressure and left ventricular end-diastolic pressure (LVEDP). However, this effect is also influenced by heart rate. Although only minimal increases in pulmonary vein pressure and LVEDP are found at a heart rate of 60 beats/minute, much larger elevations in both are observed at a heart rate of 120 beats/minute because of incomplete relaxation (Hay 2004). Therefore, both impaired relaxation and heart rate can contribute to the elevated filling pressures that produce the characteristic clinical signs and symptoms of HF. Prevalence of HFpEF (B) The prevalence of HFpEF is high; an estimated 20%–60% of patients with HF have relatively normal left ventricular ejection fraction (LVEF). The exact prevalence is unknown because of inconsistent assessment of EF and criteria used to define HFpEF. However, in prospective studies, 50% of patients with a diagnosis of HF have preserved EF. Patients with HFpEF are generally older (73–79 years) and more often are women who have hypertension. Increased Diastolic Stiffness (C) Left ventricular stiffness associated with HFpEF can be caused by a variety of factors. Regulation of collagen can produce hypertrophy of the extracellular matrix because of an imbalance between collagen type I turnover. This balance is regulated by matrix metalloproteinases, enzymes that degrade collagen, and tissue inhibitors of these enzymes. Intrinsic cardiomyocyte stiffness can also contribute to left ventricular stiffness in HFpEF in Pathophysiology (B) Mechanisms of HFpEF (C) The mechanisms of preserved and reduced HF differ considerably. In heart failure with reduced ejection fraction Table 1-1. Etiology of Heart Failure HFrEF HFpEF Non-myocardial High Output Myocardial Non-myocardia High Output Valvular disease ■■ AS ■■ AR ■■ MR ■■ MS ■■ Anemia ■■ AV fistula Hypertension CAD DM Dilated Cardiomyopathy ■■ Genetic ■■ Postpartum ■■ Viral ■■ Drug/radiation Infiltrative myopathy Valvular disease ■■ Anemia ■■ AS ■■ AV fistula ■■ AR ■■ MR ■■ MS Pericardial constraint ■■ Constriction ■■ Tamponade Myocardial Hypertension CAD DM Cardiomyopathy ■■ Genetic ■■ Postpartum ■■ Viral ■■ Drug/radiation Infiltrative myopathy AR = arrhythmia; AS = atherosclerosis; AV = arteriovenous; CAD = coronary artery disease; DM = diabetes mellitus; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; MR = mitral regurgitation; MS = mitral stenosis. Information from Heart Failure Society of America. Executive summary: HFSA 2010 comprehensive heart failure practice Guidelines. J Card Fail 2010;16:475-539. Heart Failure with Preserved Ejection Fraction 2 PSAP 2013 • Cardiology/Endocrinology Table 1-2. Comparison of Pathophysiologic Characteristics Between HFpEF and HFrEF Parameter HFpEF HFrEF LVEF Normal Decreased LVEDP Increased Increased PCWP Increased Increased Cardiac output Normal or decreaseda Normalb or decreased Stroke volume Normal or decreaseda Decreased Diastolic function Impaired Normal or impaired BNP Normal or increased Increased Neurohormonal activation Increased Increased Left ventricular wall thickness Increased Decreased Cardiac output and stroke volume may be normal at rest and abnormal with exercise. With increased heart rate. BNP = B-type natriuretic peptide; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; LVEDP = left ventricular end-diastolic pressure; PCWP = pulmonary capillary wedge pressure. Information from Barnes MM, Dorsch MP, Hummel SL, et al. Treatment of heart failure with preserved ejection fraction. Pharmacotherapy 2011;31:312-31. a b conjunction with abnormalities formed in the cytoskeletal protein titin (Borlaug 2011). Through one or more of these pathophysiologic changes, an increased passive stiffness of the left ventricle will result, leading to hemodynamic abnormalities. In patients with HFpEF, small changes in left ventricular volume are associated with relatively large changes in ventricular diastolic pressure. Lack of ventricular compliance limits the Frank-Starling mechanism; therefore, stroke volume does not substantially increase as filling pressures rise (Zile 2004). An increase in diastolic stiffness often manifests as pulmonary edema because of the high filling pressures required to achieve adequate venous return. such as left ventricular hypertrophy, endothelial dysfunction, and vascular and myocardial collagen deposition (i.e., fibrosis) (Weber 2001). Dysregulation of the autonomic system also occurs in HF as a result of baroreflex response from inadequate stroke volume. Activation of the sympathetic nervous system leads to progression of left ventricular remodeling, reflected by rises in norepinephrine concentrations with both types of HF (Kitzman 2002). Furthermore, sympathetic overactivity reduces downstream β-adrenergic responsiveness, producing chronotropic incompetence during exercise. Whether impaired heart rate response is a compensatory mechanism to improve diastolic filling during exercise is unclear. However, similar to HFrEF, patients with HFpEF are unable to adequately achieve maximal exercise tolerance; as a result, they experience dyspnea and fatigue (Barnes 2011; Phan 2010). Neurohormones (C) The renin-angiotensin-aldosterone system (RAAS) has also been implicated in the pathophysiology and progression of both HFrEF and HFpEF (Barnes 2011). Although neurohormonal activation of RAAS is well established in systolic dysfunction, less is known about its contribution to diastolic dysfunction. However, hypertension, left ventricular hypertrophy, myocardial fibrosis, and vascular dysfunction are all processes directly modulated by RAAS that are closely associated with HFpEF (Massie 2008). Furthermore, the trophic effects of increased angiotensin II activity are believed to impair the myocardium, leading to the development of HFpEF (Yamamoto 2000; Schunkert 1993). Aldosterone may also substantially contribute to the pathogenesis of HFpEF through the mineralocorticoid receptor. The effects of aldosterone activation itself can induce hypertension through sodiumwater retention and other associated HFpEF processes PSAP 2013 • Cardiology/Endocrinology Inflammation (C) Inflammation is also believed to contribute to HF development and progression (Braunwald 2008). Circulating levels of inflammatory markers such as C-reactive protein, interleukin-6, and tumor necrosis factor alpha, as well as galectin-3 (a modulator of both inflammation and fibrosis), are elevated in both types of HF and reportedly portend worse outcomes. The strong association with HFpEF may be partly explained by inflammation from common comorbidities such as hypertension and chronic heart disease and by mechanisms related to elevated left ventricular filling pressures (De Boer 2011; Kalogeropoulos 2010; Williams 2008). Despite recent advances in knowledge, more data are needed to further delineate the role of inflammation in HFpEF. 3 Heart Failure with Preserved Ejection Fraction Prognosis (B) Similar to patients with HFrEF, reports evaluating patients with HFpEF estimate a 50% mortality rate at 5 years (Vasan 1999) with comparable morbidity and hospitalization. Re-hospitalizations for HF are estimated at 50% by 6 months after discharge (Hunt 2009). Furthermore, the functional status of patients with HFpEF is similar to those of HFrEF. However, more patients with HFrEF experience function-limiting dyspnea initially at baseline (odds ratio [OR] 0.62; 95% confidence interval [CI], 0.44–0.86; p=0.004), whereas patients with HFpEF are functionally limited after 6 months (p=0.02). These data and others indicate that in addition to significant overall mortality in HFpEF, readmission and functional decline after hospitalization contribute to substantial morbidity (Smith 2003). 3.00–23.1, p<0.001), as was severe diastolic dysfunction (i.e., advanced reduction in compliance or reversible/ fixed restrictive filling) (HR 10.17 [95% CI, 3.28–31.0], p<0.001) (Redfield 2003). Given that patients with HFpEF may not always present with clinical signs and symptoms of HF, diastolic dysfunction can go unrecognized in the general population. However, even mild dysfunction is associated with a marked increase in mortality. Therefore, it is important to employ rigorous screening techniques in patients at risk of developing HFpEF. Cause of Death (C) Although noncardiovascular deaths occur more often in the HFpEF population than the HFrEF population, patients with HFpEF are still more likely to die of cardiovascular causes than noncardiovascular causes. Noncardiovascular mortality appears to be related to older age and comorbidities such as diabetes and cerebrovascular accident. According to findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial, an estimated 60% of patients with HFpEF die of cardiovascular causes such as sudden cardiac death (26%), HF (14%), and myocardial infarction (5%). Overall rates of cardiovascular death in this study were lower than in patients with HFrEF from other randomized clinical studies (Zile 2010). Differences in rates of cardiovascular death are likely caused by factors related to structural and functional abnormalities. Patients with HFpEF have diffuse and concentric remodeling, whereas patients with HFrEF usually have focal areas of fibrosis. Comorbidity Burden (C) Patients with HFrEF and HFpEF experience similar rates of overall mortality and hospitalization. However, non-HF hospitalizations and noncardiovascular deaths are higher for HFpEF, indicating that noncardiac comorbidities influence outcomes to a greater degree in these patients (Ather 2012). Hospitalized patients are generally older, obese, and anemic, and they are more likely to have hypertension and atrial fibrillation than patients with HFrEF (Owan 2006). Ambulatory patients with less advanced HFpEF are also older, anemic, and hypertensive, and they tend to have higher prevalence of diabetes mellitus, cerebrovascular accident, and chronic obstructive pulmonary disease than ambulatory patients with reduced HF (Ather 2012). In addition to the higher prevalence of comorbidities associated with HFpEF, their impact on death may be different from reduced HF. For example, chronic obstructive pulmonary disease is associated with increased mortality in patients with HF; however, the risk of death is higher with HFpEF than with HFrEF (Ather 2012). As such, treatment of these comorbidities should be underscored when managing patients with HFpEF. Risk Factors and Lifestyle Modifications (A) Because HFpEF is closely associated with advanced age, other age-related comorbidities are common. Diabetes, hypertension, obesity, kidney failure, atrial fibrillation, and anemia are observed more often with HFpEF than with HFrEF (McMurray 2008). However, coronary artery disease is more often associated with HFrEF (Ather 2012). Diabetes is a major risk factor for HFpEF, independently increasing the risk of 5-year mortality by 1.8 times (Tribouilloy 2008). Furthermore, patients with diabetes are more likely to die of cardiovascular causes than noncardiovascular causes. The risk factors for HFpEF identified from epidemiologic studies can be categorized as either modifiable (e.g., obesity, hypertension, diabetes, coronary artery disease) or nonmodifiable (e.g., advanced age, female sex) (Alagiakrishnan 2012). Patients at risk of HFpEF should be counseled by appropriate caregivers on how to reduce certain risk factors through lifestyle modifications (e.g., diet, exercise). Diastolic Dysfunction and Survival (C) A relationship between the severity of diastolic dysfunction and survival has been reported. However, only one-half of those with diastolic dysfunction show clinical symptoms. Diastolic impairment measured by simple mitral inflow patterns using Doppler echocardiography was found to be associated with a 2-fold increase in allcause mortality and a 3-fold increase in cardiac death (Bella 2002). Using data from more rigorous criteria by Doppler measurements has shown a strong association between the severity of diastolic abnormalities and survival. In a large cross-sectional survey, mild and moderate diastolic dysfunction (i.e., impaired relaxation with moderate elevation of filling pressures) were predictive of all-cause mortality (hazard ratio [HR]) 8.31; 95% CI, Heart Failure with Preserved Ejection Fraction 4 PSAP 2013 • Cardiology/Endocrinology Clinical Presentation and Diagnosis (A) pulmonary artery systolic pressures (normal 15–30 mm Hg) are common. Diastolic function is measured by two major components of mitral flow: E wave (early passive flow) and A wave (late flow with atrial kick). As diastolic dysfunction worsens, left ventricular compliance becomes further reduced, and left atrial pressure increases. These changes are detected on echocardiography as high-velocity E wave with short deceleration time (less than 150 milliseconds) and low-velocity or absent A wave. The left ventricular size is usually normal, with left ventricular hypertrophy observed in less than one-half of patients with a diagnosis of HFpEF (Zile 2002a). Biomarkers, to some extent, may help determine the prognosis when used in combination with other diagnostic assessments to guide the clinical practitioner. Natriuretic peptides are recommended to be used together with the physical examination to diagnose acute HF (HFSA 2010a; Hunt 2009; Dickstein 2008). In general, patients with both types of HF will have elevated concentrations of natriuretic peptides; however, median levels were higher with HFrEF than with HFpEF in several studies (Bursi 2006; Maisel The clinical presentation of HF may be indistinguishable between HFpEF and HFrEF (Table 1-3). Although the presence of HF could be determined given the history, physical examination, electrocardiography, or chest radiography, further testing by echocardiography is required. Several criteria have been proposed for the diagnosis of HFpEF, but a lack of consensus has prevented the establishment of a universally accepted approach. Practicing cardiologists commonly diagnose HFpEF using signs and symptoms of congestive HF as specified by the Framingham criteria (McKee 1971) and defined by the presence of an EF of 50% or greater on echocardiography within 72 hours of presentation (Gandhi 2001). (These criteria are loosely interpreted by those used in actual clinical trials.) Differential diagnoses for HFpEF are presented in Box 1-1. Echocardiography could also provide other findings consistent with HFpEF that would serve to confirm the diagnosis. Left atrial dilation is often a result of increased left atrial pressures (normal 4–12 mm Hg), and elevated Table 1-3. Comparative Incidence of Symptoms and Signs in HFpEF vs. HFrEFa Dyspnea on exertion Paroxysmal nocturnal dyspnea Orthopnea Physical examination Jugular venous distension Rales Displaced apical impulse S3 S4 Hepatomegaly Edema Chest radiography Cardiomegaly Pulmonary venous hypertension Incidence in HFpEF (%) 62–85 55 60 Incidence in HFrEF (%) 63–96 50 73 26–35 65–72 50 45 45 15 30–68 33–56 63–70 60 65 66 16 40–62 90 75 96 80 Box 1-1. Differential Diagnoses of HFpEF ■■ ■■ ■■ ■■ ■■ ■■ ■■ ■■ ■■ ■■ ■■ Atrial myxoma Chronic pulmonary disease with right HF Diastolic dysfunction of uncertain origin Episodic or reversible LV systolic dysfunction HF associated with high metabolic demand Incorrect diagnosis of HF Incorrect measurement of LVEF Obesity Pericardial constriction Primary valvular disease Pulmonary hypertension, associated with pulmonary vascular disorders ■■ Restrictive cardiomyopathies (amyloidosis, sarcoidosis, hemochromatosis) ■■ Severe hypertension, myocardial ischemia HF = heart failure; HFpEF = heart failure with preserved ejection fraction; LV = left ventricular; LVEF = left ventricular ejection fraction. Information from Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:e391-479. HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction. a HFpEF with EF > 50%; HFrEF with EF < 50%. Data represented as percentage of patients in each group. Information from Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure. Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 2002a;105:1387-93; and Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure. J Am Coll Cardiol 2007;50:768-77. PSAP 2013 • Cardiology/Endocrinology 5 Heart Failure with Preserved Ejection Fraction Diuretics (B) Through different mechanisms, both patients with HFrEF and patients with HFpEF experience symptoms of volume overload caused by elevated left ventricular filling pressures. Decreasing total fluid volume would therefore reduce left ventricular pressure and improve patient symptoms. Diuretic therapy, which can reduce filling pressures, has been reported to improve quality of life in patients with HFpEF. In one study, 150 patients with an LVEF greater than 45% were randomized to receive diuretics alone, diuretics plus irbesartan, or diuretics plus ramipril. At 52 weeks, quality-of-life scores were improved from baseline in all three groups (46%, 51%, and 50%, respectively; p<0.01 for all). Furosemide was used most often and improved patient symptoms without additive benefit from irbesartan or ramipril (Yip 2008). Dosing of diuretics is extremely important in these patients because small decreases in diastolic volume can produce relatively large reductions in blood pressure and cardiac output. Therefore, diuretics should be initiated at the lowest possible dose, together with careful monitoring of blood pressure to avoid orthostatic hypotension (Zile 2002b). In addition to symptomatic management, diuretic therapy can be used to prevent HFpEF and HFrEF in hypertensive patients. Medical management of hypertension in the elderly reduces HF risk by about 50% (Beckett 2008; Kostis 1997). The development of HFpEF after treatment with chlorthalidone, a thiazide diuretic, was compared with that of other antihypertensive drugs in an analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). This trial enrolled more than 42,000 patients older than 55 years with hypertension and more than one risk factor for coronary artery disease. Chlorthalidone reduced the risk of new-onset HFpEF better than amlodipine (HR 0.69; 95% CI, 0.53–0.91; p=0.009), lisinopril (HR 0.74; 95% CI, 0.56–0.97; p=0.032), or doxazosin (HR 0.53; 2003). Increased concentrations of N-terminal pro–B-type natriuretic peptide (NT-proBNP) have also been reported in HFpEF; however, natriuretic peptides tend to be less predictable in this population (Anand 2011). Attempts have been made to use cut points (e.g., NT-proBNP greater than 220 pg/mL, BNP greater than 200 pg/mL) for diagnosing HFpEF in the European guidelines (Paulus 2007). One potential explanation for the unpredictability of these biomarkers is that both BNP and NT-proBNP are influenced by age and sex, as well as obesity and other comorbidities closely associated with HFpEF (Packer 2011). Increased concentrations may not directly reflect the severity of HF or diastolic dysfunction, but rather, the comorbidities themselves. Therefore, natriuretic peptide concentrations are less useful in this population. Other researchers have studied biomarkers that specifically target adverse remodeling through myocardial fibrosis, a key pathway in the pathophysiology of HFpEF. Peripheral collagen markers (i.e., procollagen type I amino-terminal peptide, procollagen type III aminoterminal peptide, osteopontin) were not independently associated with clinically significant end points in this population; however, the change in osteopontin over time was predictive of all-cause mortality (Krum 2011). These preliminary findings appear to support the role of fibrosis in HFpEF. Future studies from I-PRESERVE will provide more data about other profibrotic markers (i.e., galectin-3, matrix metalloproteinase-9). Treatment (A) Clinical studies showing solid evidence in support of medical management of HFpEF are lacking. Further studies directed at the pathophysiology, biomarkers, and therapies of pathways affecting the prevention and progression of diastolic dysfunction are needed. With this limitation, current guidelines have mainly focused on improving patient symptoms and associated comorbidities. Table 1-4 summarizes current treatment modalities. Table 1-4. Treatment of HFpEF According to Clinical Signs and Pathologic Characteristics Reduce LV volume and edema Diuretics, reduce salt, nitrates Treat systolic hypertension Diuretics, β-blockers, CCBs, ACEIs, ARBs, MRAs Reverse LVH Antihypertensive agents (e.g., ARBs, diuretics) Prevent ischemia β-Blockers, CCBs, nitrates Rate control or maintenance of sinus rhythmwith AF β-Blockers, CCBs, amiodarone Prevent fibrosis ACEIs, ARBs, MRAs Increase vascular compliance and myocardial relaxation ACEIs ACEI = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin receptor blocker; CCB = calcium channel blocker (nondihydropyridines); HFpEF = heart failure with preserved ejection fraction; HR = heart rate; LVH = left ventricular hypertrophy; MRA = mineralocorticoid receptor antagonist. Heart Failure with Preserved Ejection Fraction 6 PSAP 2013 • Cardiology/Endocrinology ACE Inhibitors/ARBs/Mineralocorticoid Antagonists (B) Activation of the RAAS is involved in the pathophysiology and progression of HF. Drugs used to target this pathway have shown significant reductions in mortality and are considered cornerstone therapy for chronic HF management in patients with HFrEF. However, the evidence supporting angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) is less clear with HFpEF, despite a strong association between RAAS and both left ventricular hypertrophy and myocardial fibrosis. Several clinical trials have investigated the effects of RAAS inhibition in HFpEF and produced inconsistent results (Table 1-5). The Perindopril in Elderly People 95% CI, 0.38–0.73; p<0.001) (Davis 2008). Furthermore, risk of hospitalization was also reduced with chlorthalidone compared with amlodipine (HR 0.69;95% CI, 0.59-0.94; p=0.009), lisinopril (HR 0.74; 95% CI, 0.56-0.97; p=0.032), or doxazosin (HR 0.53; 95% CI, 0.38-0.73; p<0.001). These findings show the benefit of thiazides in hypertension management and their importance for the potential to prevent hospitalizations or the development of HFpEF. Thiazides are a reasonable choice for the initial management of hypertension; however, their preventive and therapeutic benefits must be carefully weighed against the potential advantage of loop diuretics for patients with kidney insufficiency or pulmonary congestion when greater diuretic effect is required. Table 1-5. RAAS Inhibitor Trials of ACE Inhibitors and ARBs in Patients with Heart Failure with Preserved Ejection Fraction Study/Treatment Population Duration CHARM-Preserved Candesartan vs. placebo (Yusuf 2003) 3023 patients with NYHA II–IV and EF > 40% 36.6 months Reduction in CV death or admission to hospital for CHF HR 0.86; 95% CI, 0.74–1.0; p=0.051 with fewer patients with first CHF admissions (230 vs. 279, p=0.017) Significant reductions in composite outcome of non-fatal MI and nonfatal stroke (HR 0.86; 95% CI, 0.75–0.99; p=0.037) I-PRESERVE Irbesartan or placebo (Massie 2008) 4128 patients at least 60 years of age with NYHA class II–IV and EF ≥45% 49.5 months No significant difference in death or hospitalization for cardiovascular cause HR 0.95; 95% CI, 0.86–1.05; p=0.35 PEP-CHF Perindopril or placebo (Cleland 2006) 850 patients at least 70 years of age, wall motion index < 1.4 (EF ≤ 40%) treated with diuretics 2.1 years Reduction in mortality (HR 0.692; 95% CI, 0.474–1.010; p=0.055) and significant reduction for heart failure hospitalizations (HR 0.628; 95% CI, 0.408–0.966; p=0.033) Improvements in functional class and 6-minute walk distance with perindopril Quinapril or placebo (Zi 2003) 74 elderly patients (mean age 78 years) with NYHA II–III) 6 months No differences in 6-minute walk distance or QOL scores Losartan or placebo (Warner 1999) 20 patients (mean age 64 years) with EF > 50% 2 weeks Exercise time increased to 12.3 minutes vs. 11.3 minutes at baseline, and placebo (p<0.05 for both) QOL improved with losartan vs. placebo (18 vs. 22, p<0.05) 38 weeks No difference in blood pressure reduction or diastolic relaxation velocity between groups Valsartan In Diastolic 198 patients with Dysfunction (VALIDD) hypertension with Valsartan or placebo LVEF > 50% (Solomon 2007) Significant Outcomes ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CHF = congestive heart failure; CV = cardiovascular; EF = ejection fraction; HR = heart rate; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NYHA = New York Heart Association; QOL = quality of life; RAAS = renin-angiotensin-aldosterone system. Information from Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved leftventricular ejection fraction: the CHARM-Preserved trial. Lancet 2003;362:777-81; Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456-67; Cleland JGF, Tendera M, Adamus J, et al. The Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) study. Eur Heart J 2006;27:2338-45; Zi M, Carmichael N, Lye M. The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovasc Drugs Ther 2003;17:133-9; Warner JG, Metzger C, Kitzman DW, et al. Losartan improves exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise. J Am Coll Cardiol 1999;33:1567-72; and Solomon SD, Janardhanan R, Verma A, et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomized trial. Lancet 2007;369:2079-87. PSAP 2013 • Cardiology/Endocrinology 7 Heart Failure with Preserved Ejection Fraction with Chronic Heart Failure (PEP-CHF) study and Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity–Preserved study (CHARMPreserved) trials showed marginal to moderate decreases in death or hospitalization for HF using RAAS antagonists; however, I-PRESERVE resulted in no significant difference in death or hospitalization. Important differences in trial design and study enrollment may explain these disparities. Because of inadequate enrollment and high attrition rates, PEP-CHF was severely underpowered, limiting the interpretation of data. Furthermore, both CHARM-Preserved and PEP-CHF enrolled patients with an EF greater than 40%—a less rigorous criterion for HFpEF by current standards. The I-PRESERVE trial was more appropriately powered, used a stricter criteria (EF of 45% or greater), and resulted in less impressive results. Despite conflicting findings from these three major trials, the totality of evidence at present suggests that ACE inhibitors and ARBs are not effective treatment for HFpEF. Targeting the mineralocorticoid receptor using mineralocorticoid receptor antagonists (e.g., spironolactone, eplerenone) may hold greater promise as an effective strategy for HFpEF. Aldosterone blockade been shown in experimental studies to reduce myocardial fibrosis; decrease inflammation; and improve vascular compliance, endothelial dysfunction, and myocardial perfusion. Aldosterone may also improve collagen markers as well as clinical end points in patients with HFpEF (Desai 2011; Daniel 2009; Mak 2009; Roongsritong 2005; Mottram 2004). Several small studies have investigated changes in echocardiographic parameters to detect clinical improvements in diastolic function. One study showed that treatment with eplerenone for 12 months produced no difference in diastolic function parameters other than greater deceleration time compared with control (−82 milliseconds vs. −7 milliseconds; p=0.032) and only modest functional improvements (Mak 2009). Elevations in peripheral collagen marker procollagen type III amino-terminal peptide were attenuated during treatment, suggesting a reduction in collagen turnover; this supports eplerenone treatment as a key mechanism in improving function in these patients. Spironolactone has also shown benefit in symptomatic patients with HFpEF (Mottram 2004). Patients randomized to 6 months of treatment showed significant improvement of left ventricular long-axis systolic function, as characterized by increases in long-axis strain rate, peak systolic strain, and regional myocardial systolic function. Of importance, these findings were independent of its antihypertensive effects, supporting a direct relationship between aldosterone blockade and diastolic function. The effects of spironolactone are currently being investigated in the Treatment of Preserved-Cardiac-Function Heart Failure (TOPCAT) trial, a large randomized doubleblind study of more than 3000 patients with HFpEF (Desai 2011). This study enrolled patients who are symptomatic, who are 50 years or older, whose LVEF is 45% or greater, Heart Failure with Preserved Ejection Fraction and who have been either hospitalized for HF within the previous year or have elevated natriuretic peptides. The primary composite end point is cardiovascular death, HF hospitalization, or aborted cardiac arrest, although morbidity and quality of life, biomarkers, and echocardiographic changes will be evaluated as well. The results of this study should provide more conclusive data about the role of mineralocorticoid receptor antagonists in HFpEF. β-Blockers (B) β-Blockers have proven benefits in patients with systolic dysfunction, but less is known about their use in patients with HFpEF. Because tachycardia may unmask clinical signs and symptoms of HF in patients with HFpEF, identifying therapy to reduce such symptoms seems warranted. Rapid heart rates increase myocardial oxygen demand and can promote ischemia. Furthermore, shortened diastole as a result of HFpEF can either increase diastolic pressure without improving diastolic volume or produce increased heart rate and thereby worsen HF (Zile 2002a). Therefore, β-blocker use could theoretically be used to maintain resting heart rates between 60 beats/minute and 70 beats/ minute to control tachycardia and promote diastolic filling, which has potential to improve outcomes in patients with HFpEF. The theoretical potential of β-blockade to improve clinical outcomes in patients with HFpEF led to several trials (Table 1-6). Benefit in patients with HFpEF was first investigated in a study of 158 patients with prior myocardial infarction, LVEF of 40% or greater, and pulmonary congestion after treatment with diuretics and an ACE inhibitor (Aronow 1997). Patients were randomized to either control group or propranolol. After 1 year of propranolol treatment, LVEF was significantly increased from baseline, and a greater reduction in left ventricular mass compared with control was also observed. Furthermore, a 35% reduction in total deaths and 37% reduction in composite end point of total mortality or nonfatal myocardial infarction was observed after 32 months of propranolol therapy. A major limitation to this study is that the criteria for LVEF were less strict than in studies performed more recently. Nevertheless, this study was the first to show a mortality benefit with β-blockade in HFpEF. In a more recent study, 113 symptomatic patients with an LVEF of 45% or greater were randomized to either carvedilol or placebo in addition to standard therapy, and echocardiographic parameters were assessed (Bergstrom 2004). Although most parameters did not show a difference between groups, there was a significant improvement in mitral flow E/A ratio with carvedilol driven by left ventricular relaxation. This effect was more pronounced with higher heart rates, a condition that can produce an increase in myocardial oxygen demand and a reduction in exercise capacity in patients with HFpEF. The potential effects of β-blockers on mortality were also evaluated in elderly patients with HFpEF. The SENIORS 8 PSAP 2013 • Cardiology/Endocrinology Table 1-6. Trials of β-Blockers in Patients with HFpEF Duration (months) Study/Treatment Population Significant outcomes Atenolol or nebivolol (Nodari 2003) 30 hypertensive patients with NYHA II or III and EF ≥ 50% with evidence of diastolic function 6 Both atenolol and nebivolol significantly decreased improved hemodynamic parameters (CI, SV, BP, HR) and E/A ratio at 6 months vs. baseline; however, nebivolol also reduced mPAP and PCWP BHAT trial Propranolol or control (no propranolol) (Aronow 1997) 158 patients at least 62 years of age with NYHA class II or III CHF and EF ≥ 40% after diuretics and ACEI for 2 months 12 Greater increase in LVEF (p<0.0001) and reduction in LV mass (p=0.0001) with propranolol vs. control at 1 year Propranolol significantly reduced total mortality by 35% (p=0.007) and combined end point of total mortality and nonfatal MI by 37% (p=0.002) Carvedilol or standard therapy (Takeda 2004) 40 patients with mildmoderate HF and EF ≥ 45% 12 Carvedilol improved NYHA functional class from 2.37 to 1.56 (p<0.01), BNP from 175 to 106 pg/mL (p<0.01), and exercise capacity 4.75 to 5.68 METs (p<0.02). No significant changes were observed with the conventional treatment SWEDIC Carvedilol or placebo (Bergstrom 2004) 113 symptomatic patients with normal systolic LV function and diastolic dysfunction 6 No significant difference in mitral flow E/A ratio, deceleration time, isovolumic relaxation time, and systolic/diastolic pulmonary venous flow velocity during study Significant improvement in E/A ratio at study end with carvedilol vs. placebo (0.72–0.83 vs. 0.71–0.76, p<0.05) SENIORS Nebivolol in impaired EF vs. preserved HF Substudy of 2111 patients with symptomatic HF with either reduced or reduced EF (35 ≥ vs. < 35%) 21-month follow-up Nebivolol vs. placebo on all-cause mortality or cardiovascular hospitalizations was 0.81 (95% CI, 0.63–1.04) in preserved EF (p=0.720). No significance differences between nebivolol vs. placebo between reduced vs. preserved EF groups ACEI = angiotensin-converting enzyme inhibitor; BNP = B-type natriuretic peptide; CHF = congestive heart failure; CI = cardiac index; E/A ratio = E/A wave = early passive flow/late flow with atrial kick; HFpEF = HF with preserved EF; HFrEF = HF with reduced EF; HR = heart rate; LVEF = left ventricular ejection fraction; LVEDP = left ventricular end-diastolic pressure; MET = metabolic equivalent; MI = myocardial infarction; mPAP = mean pulmonary artery pressure; NYHA = New York Heart Association; PCWP = pulmonary capillary wedge pressure; SV = stroke volume. Information from Nodari S, Metra M, Cas LD. Β-blocker treatment of patients with diastolic heart failure and arterial hypertension. A prospective, randomized, comparison of the long-term effects of atenolol vs. nebivolol. Eur J Heart Fail 2003;5:621-7; Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction ≥ 40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol 1997;80:207-9; Takeda Y, Fukutomi T, Suzuki S, et al. Effects of carvedilol on plasma b-type natriuretic peptide concentration and symptoms in patients with heart failure and preserved ejection fraction. Am J Cardiol 2004;94:448-53; and Bergstrom A, Andersson B, Edner M, et al. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-Echocardiographic Study (SWEDIC). Eur J Heart Fail 2004;6:453-61. trial was a randomized, double-blind, placebo-controlled, parallel-group trial evaluating the effect of nebivolol on all-cause mortality or cardiovascular hospitalizations as a composite end point. The substudy analyzed 752 elderly patients (70 years and older) with an EF greater than 35% (defined as HFpEF) versus 1359 patients with an EF of 35% or less (defined as HFrEF) (van Veldhuisen 2009). The mortality rates at 21 months were similar (31% in patients with HFpEF, 34% in patients with HFrEF). Compared with placebo, the hazard ratio for nebivolol in HFpEF was 0.81 (95% CI, 0.63–1.04; p=0.720). These findings suggest that nebivolol showed similar effects on mortality in both categories of HF. However, the findings are limited given the retrospective study design and less rigorous definition of HFpEF. PSAP 2013 • Cardiology/Endocrinology Large randomized clinical trials are still needed to determine the benefit of β-blockers on outcomes in patients with HFpEF. However, given the relationship between heart rate and filling times, β-blockers may be a reasonable option in patients with elevated heart rates and a comorbidity in which β-blocker therapy may be indicated (e.g., hypertension). Calcium Channel Antagonists (B) Nondihydropyridine calcium channel blockers (e.g., diltiazem, verapamil) have negative inotropic and chronotropic effects on the myocardium that can promote relaxation and improve diastolic filling (Table 1-7). In a small study, 20 symptomatic patients with HFpEF were randomized to 5 weeks of verapamil or placebo; results 9 Heart Failure with Preserved Ejection Fraction Table 1-7. Trials of Calcium Channel Blockers, Digoxin, Diuretics, and Mineralocorticoid Receptor Antagonists in Patients with HFpEF Study/Treatment Population Calcium Channel Antagonists Verapamil or placebo 20 men with EF > 45% (Setaro 1990) and diastolic dysfunction Verapamil or placebo (Hung 2002) Digoxin DIG post hoc analysis (Ahmed 2006) DIG substudy analysis (Meyer 2008) Diuretics Diuretics, irbesartan, and ramipril (Yip 2008) Significant Outcomes 5-week crossover trial Verapamil significantly improved exercise capacity from baseline ([3.4–13.9 minutes] and filling rate [1.85–2.29 edv/second]; all p<0.05); placebo was not significantly different Verapamil improved CHF and LV diastolic function; increased tolerance to exercise. Results showed a reduced CHF score (from 5.6 to 3.5), decreased isovolumic relaxation time (from 84 to 73 milliseconds), and increased exercise time (from 7.4 to 8.3 minutes); p<0.05 for all) compared with baseline 15 elderly patients with 3-month crossover trial NYHA II–III and diastolic (1-week washout) dysfunction 5548 HFrEF and HFpEF patients from the DIG trial randomized to digoxin or placebo with serum digoxin concentration at 1 month 916 pairs of patients with systolic and diastolic HF 40-month median follow-up 150 patients with HF, EF > 45% randomized to one of three treatment groups QOL scores at 52 weeks, echocardiographic and hemodynamic parameters at 1 year QOL scores improved in all three groups. LVEF was unchanged at 1 year, but diuretics with irbesartan or ramipril improved LV function and lowered NT-proBNP 4 months Spironolactone improved E/A ratio (0.71 to 0.84, p=0.025) and deceleration time (285 to 230, p=0.035) compared with baseline Eplerenone did not significantly affect pro-collagen type III and I aminoterminal peptides, MMP-2, IL-6 and IL-8, and tumor necrosis factor-α at 6 months, but significantly attenuated pro-collagen type III at 12 months (p=0.006) Spironolactone improved myocardial function via increased long-axis strain rate (−1.57 to −1.91 s-1, p<0.01), peak systolic strain (−20.3% to −26.9%, p<0.001), qualitative assessment of regional systolic function (7.4 to 8.6 dB, p=0.08), and reduced posterior wall thickness (p=0.04) Mineralocorticoid Receptor Antagonists Spironolactone or 30 elderly subjects placebo with isolated diastolic (Roongsritong 2005) dysfunction Eplerenone or 44 elderly patients with control HFpEF (Mak 2009) Spironolactone or placebo (Mottram 2004) Duration 30 hypertensive patients with dyspnea, EF > 50%, and diastolic dysfunction 2-year follow-up 6 months 6 months Serum digoxin concentration of 0.5–0.9 ng/mL associated with lower all-cause mortality compared with placebo (HR 0.77; 95% CI, 0.67–0.89; p<0.0001), CV mortality (HR 0.83; 95% CI, 0.71–0.97; p=0.019), and HF mortality (HR 0.63; 95% CI, 0.49–0.82; p<0.0001) Type of HF (EF > 45%) did not influence results Compared with placebo, digoxin decreased HF hospitalization in HFrEF (HR 0.73; 95% CI, 0.54–0.97; p=0.033) and HFpEF (HR 0.64; 95% CI, 0.45–0.90; p=0.010) CHF = congestive heart failure; CV = cardiovascular; E/A ratio = E/A wave = early passive flow/late flow with atrial kick; edv = end-diastolic volume; EF = ejection fraction; HFpEF = heart failure with preserved ejective fraction; HFrEF = HF with reduced EF; HR = heart rate; IL = interleukin; LV = left ventricular; MMP = matrix metalloproteinase; mPAP = mean pulmonary artery pressure; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NYHA = New York Heart Association; QOL = quality of life. Information from Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol 1990;66:981-6; Hung MJ, Cherng WJ, Kuo LT, et al. Effect of verapamil in elderly patients with left ventricular diastolic dysfunction as a cause of congestive heart failure. Int J Clin Pract 2002;56:5762; Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006;27:178-86; Meyer P, White M, Mujib M, et al. Digoxin and reduction of heart failure hospitalization in chronic systolic and diastolic heart failure. Am J Cardiol 2008;102:1681-6; Yip GW, Wang M, Wang T, et al. The Hong Kong diastolic heart failure study: a randomized controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 2008;94:573-80; Roongsritong C, Sutthiwan P, Bradley J, et al. Spironolactone improves diastolic function in the elderly. Clin Cardiol 2005;28:484-7; Mak GJ, Ledwidge MT, Watson CJ, et al. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol 2009;54:1674-82; and Mottram PM, Haluska B, Leano R, et al. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation 2004;110:558-65. Heart Failure with Preserved Ejection Fraction 10 PSAP 2013 • Cardiology/Endocrinology showed a 33% relative improvement in exercise capacity and peak filling rate compared with baseline (Hung 2002). Functional improvement was further supported by another small study showing increased exercise time and reduced isovolumic relaxation time with verapamil after 3 months (Setaro 1990). Together, these results suggest clinical benefit and therapeutic efficacy to improve exercise tolerance in elderly patients with HFpEF, although no morbidity or mortality data exist on these agents. Given their ability to reduce heart rate, they can also be used as an alternative to β-blockers in patients with elevated heart rates and comorbidities when calcium channel blockers are indicated. predispose the patient to other life-threatening arrhythmias. Both amiodarone and dronedarone are used to treat tachyarrhythmias in patients with underlying structural heart disease, and both have been studied in this population. The effect of amiodarone in patients with HFpEF was first examined in the Basel Antiarrhythmic Study of Infarct Survival trial. There were 312 patients (primarily men) with arrhythmias and HFpEF (mean EF 43%); 41% of the study group had previous myocardial infarction. Results from this study showed 5% mortality in the treatment group compared with 13% in the control group at 1 year, suggesting that amiodarone reduces sudden death in this population (Burkart 1990). Long-term outcomes in 359 patients with HFpEF were further supported in a subanalysis of the Polish amiodarone trial, where this agent reduced sudden cardiac death at 46 months when compared to placebo (Budaj 1996). The effects of dronedarone on outcomes are discordant with those of amiodarone. The Permanent Atrial fibriLLAtion outcome Study using Dronedarone on top of standard therapy (PALLAS) investigated dronedarone use in addition to standard therapy, but it was terminated early because of safety concerns in this population. A total of 3236 patients with atrial fibrillation were randomized to dronedarone or placebo. Subgroup analysis showed an increased risk of cardiovascular hospitalization or death, which was significant for patients with HFrEF (HR 2.17; 95% CI, 1.15–4.070) and for patients with HFpEF (HR 1.61; 95% CI, 1.89–2.64) (Connolly 2011). Therefore, dronedarone should be avoided in patients with permanent atrial fibrillation and HF. These dissimilar effects between antiarrhythmics in patients with HFpEF appear to support a role for amiodarone but not for dronedarone. Although limited data suggest potential benefit of amiodarone on sudden cardiac death in select patients, there are profound safety concerns with dronedarone use. Therefore, dronedarone should be used selectively and with caution in all patients with HF. Digoxin (B) In addition to its positive inotropic effects, digoxin reduces the neurohormonal activation associated with the pathophysiology of HF. By inhibiting the sodiumpotassium ATPase pump, this agent exerts a vagomimetic effect not only on the heart but also the kidney, which in turn reduces activity of the sympathetic nervous system and RAAS (Meyer 2008) Whether this improvement by digoxin is regulated through baroreceptor function remains to be established in HFpEF. Clinical studies suggest the possible benefit of digoxin for HFpEF. In a post hoc analysis of the Digitalis Investigation Group (DIG) trial, 1687 patients with both types of HF who received digoxin were compared with 3861 patients who received placebo. The analysis revealed that serum digoxin concentrations of 0.5–0.9 ng/mL reduced mortality in all patients with HF, including those with HFpEF, thereby expanding the potential indication of digoxin to be used for both categories of HF. Furthermore, this study supports standard digoxin therapeutic ranges (less than 0.9 ng/mL) for HFrEF as well as HFpEF (Ahmed 2006). In a more recent analysis of patients with HFpEF from the DIG trial (Meyer 2008), there was a significant risk reduction in the combined end point of HF hospitalization or HF mortality in those receiving digoxin at 2 years (HR 0.69; 95% CI, 0.50–0.95; p=0.025), but this was not significant at 3.2 years (HR 0.79; 95% CI, 0.60–1.03; p=0.085). Improvements in the combined end point were likely driven by improved HF hospitalization. Given the high costs associated with hospitalization, these findings support the role of digoxin in HFpEF. Despite these preliminary findings, further randomized clinical trials are needed to establish the agent’s efficacy in HFpEF. At present, no guideline recommendations exist for using digoxin to treat HFpEF. However, digoxin can be considered on a case-by-case basis, especially in patients with frequent hospitalizations for HFpEF. Statins (B) Statins produce a variety of effects independent of low-density lipoprotein cholesterol reduction. Several of these beneficial effects may prevent or improve HFpEF. In addition to antifibrotic effects observed in experimental studies, statins can increase arterial distensibility and reduce blood pressure in hypertensive patients. These effects could decrease fibrosis, left ventricular mass, and afterload in patients with HFpEF. Furthermore, statins may reduce adverse remodeling after myocardial infarction and therefore may play a preventive role in the development of diastolic dysfunction. Statins may also have potential benefits on endothelial function and inflammation (Fukuta 2005). Amiodarone/Dronedarone (B) Conditions such as myocardial infarction and HF can increase the risk of sudden cardiac death and may PSAP 2013 • Cardiology/Endocrinology 11 Heart Failure with Preserved Ejection Fraction Statin mortality benefit in HFpEF was first reported in a preliminary study that evaluated 137 patients with HF and an EF of 50% or greater (Fukuta 2005). Patients receiving statin therapy in addition to standard therapy had a lower risk of death than those not receiving statins (relative risk [RR] 0.22; 95% CI, 0.07–0.64; p=0.006). A retrospective study of 13,533 older patients with HFpEF reported that statin therapy reduced 1- and 3-year mortality rates (RR 0.69; 95% CI, 0.61–0.78) compared with nonstatin therapy (RR 0.73; 95% CI, 0.68–0.79). These results were still significant after adjusting for total cholesterol, coronary artery disease, diabetes mellitus, hypertension, and age (Shah 2008). Other study data appear to support these conclusions (Tehrani 2010). According to current data and experimental studies, statins appear to show benefits in patients with HFpEF. Further prospective randomized studies are warranted to determine whether statins should be standard therapy for treating patients with HFpEF. by improving left ventricular mass, diastolic function, and left ventricular remodeling (Little 2005; Thohan 2005). Another novel product specifically targeting the HFpEF population was recently evaluated in the PARAMOUNT trial (Prospective comparison of ARNI and ARB on Management Of heart failure with preserved ejection fraction), a randomized, parallel-group, double-blind trial of 149 patients comparing LCZ696 versus valsartan alone. The LCZ696 is a combination of valsartan and AH4377, a neprilysin inhibitor that inhibits natriuretic peptide and angiotensin degradation. Through the accumulation of ANP (atrial natriuretic peptide), BNP, and CNP (C-type natriuretic peptide), cyclic guanosine monophosphate is attenuated, producing vasodilation and modest improvements in structural cardiac abnormalities. At 12 weeks, systolic blood pressure and NT-proBNP were significantly reduced in patients receiving LCZ696 compared with valsartan (9.3 vs. 2.9 mm Hg, p=0.001; and 605 vs. 835 pg/ mL, p=0.005, respectively). Furthermore, left atrial size was reduced at 36 weeks, suggesting that LCZ696 lowers the risk of associated HF events (Solomon 2012). These surrogate markers suggest a potential benefit for patients with HFpEF, but larger prospective studies are needed to determine whether LCZ696 provides significant benefit on clinical outcomes. Future Therapies (B) Several drugs have been investigated in experimental studies and preclinical trials. Sildenafil reduces chamber size, pressure-induced ventricular hypertrophy, and fibrosis (Westermann 2009; Takimoto 2005). Ivabradine improves diastolic left ventricular function, reduces hypoxia and remodeling, and increases nitric oxide bioavailability through heart rate reduction in rats (Fang 2012). Ivabradine is currently approved for HF in Europe, but clinical studies have exclusively focused on HFrEF. As an inhibitor of late sodium current in cardiac myocytes, ranolazine could reduce intracellular calcium, leading to improved myocardial relaxation. A small, randomized, prospective single-center trial is enrolling patients to determine whether ranolazine can provide clinical benefit in patients with HFpEF (Jacobshagen 2011). Advanced glycation end-products are formed through proteins and sugars to produce excessive crosslinks in the myocardium and vasculature. These changes can lead to diastolic dysfunction. Alagebrium, an advanced glycation end-product crosslink breaker, was studied in patients with HFpEF and showed promising preliminary results Guideline-Based Assessment and Treatment Plan (B) Several papers discuss the theoretical potential of standard HF drugs in the treatment of HFpEF, but clinical evidence supporting efficacy is currently lacking. Therefore, guidelines from the American College of Cardiology/American Heart Association (Jessup 2009; Hunt 2005), Heart Failure Society of America (HFSA 2010a), and European Society of Cardiology (McMurray 2012) focus on the diagnosis of HFpEF and recommend empiric strategies for relieving signs and symptoms of HF and treating associated comorbidities (Table 1-8). In addition to blood pressure monitoring (goal less than 130/80 mm Hg) and a low-sodium diet (2–3 g/ day), the use of a thiazide and/or loop diuretic as needed is recommended for patients with clinical evidence of hypervolemia and congestion. Because these patients are generally more sensitive to diuresis, blood pressure and kidney function should be carefully monitored. An ACE Table 1-8. ACC/AHA Guidelines for the Treatment of HFpEF ACC/AHA Guidelines Class 1 Recommendations Treatment Modalities Control blood pressure < 130/80 mm Hg No specified antihypertensive agent Prevent tachycardia or rate control in AF Digoxin, BBs, CCBs, amiodarone Reduce central blood volume Low-salt diet, diuretics, nitrates ACC/AHA = American College of Cardiology/American Heart Association; AF = atrial fibrillation; BB = β-blocker; CCB = calcium channel blocker; HFpEF = heart failure with preserved ejection fraction. Heart Failure with Preserved Ejection Fraction 12 PSAP 2013 • Cardiology/Endocrinology inhibitor should also be considered, especially in symptomatic patients with HFpEF who have atherosclerotic cardiovascular disease or diabetes and one additional risk factor (e.g., hypertension). An ARB should be considered in patients who cannot tolerate ACE inhibitors. β-Blockers are recommended for patients with prior myocardial infarction, history of hypertension, or atrial fibrillation requiring ventricular rate control. β-Blockers may be an even stronger consideration in treating comorbidities such as hypertension in patients with elevated heart rates. For patients in whom calcium channel blockers are indicated (e.g., symptomatic angina or hypertension), nondihydropyridine calcium channel blockers (e.g., diltiazem, verapamil) are recommended because of their effect on the myocardium and heart rate. These calcium channel blockers may also be indicated in patients who would be candidates for β-blockers but are intolerant of these agents. Digoxin is also a less well-established option for patients who are frequently hospitalized (HFSA 2010a; Hunt 2009; Dickstein 2008). Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction ≥ 40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol 1997;80:207-9. PubMed Link Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998-1005. PubMed Link Barnes MM, Dorsch MP, Hummel SL, et al. Treatment of heart failure with preserved ejection fraction. Pharmacotherapy 2011;31:312-31. PubMed Link Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887-98. PubMed Link Bella JN, Palmieri V, Roman MJ, et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation 2002;105:1928-33. PubMed Link Conclusion (A) Because HFpEF is still not well understood, more research is needed to better understand the pathophysiology and progression of this clinical syndrome. Patient diagnosis is primarily based on clinical symptoms and echocardiography, although biomarkers may have an emerging future role. Agerelated diseases are commonly observed with HFpEF, and treatment should be targeted to reduce comorbidity burden. Although experimental studies appear to support a pathophysiologic and mechanistic rationale for therapy, strong clinical evidence is lacking. Therefore, current guidelines are largely empiric, focusing on patient signs and symptoms of HF and standard therapies used for reduced EF. Several large randomized clinical studies are investigating both standard and novel agents for HFpEF. Bergstrom A, Andersson B, Edner M, et al. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-Echocardiographic Study (SWEDIC). Eur J Heart Fail 2004;6:453-61. PubMed Link Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011;32:670-9. PubMed Link Braunwald E. Biomarkers in heart failure. N Engl J Med 2008;358:2148-59. PubMed Link Budaj A, Kokowicz P, Smielak-Korombel W, et al. Lack of effect of amiodarone on survival after extensive infarction. Polish Amiodarone Trial. Coron Artery Dis 1996;7:315-9. PubMed Link References Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006;27:178-86. PubMed Link Burkart F, Pfisterer M, Kiowski W, et al. Effect of antiarrhythmic therapy on mortality in survivors of myocardial infarction with asymptomatic complex ventricular arrhythmias: Basel antiarrhythmic study of infarct survival (BASIS). J Am Coll Cardiol 1990;16:1711-8. PubMed Link Alagiakrishnan K, Banach M, Jones LG, et al. Update on diastolic heart failure or heart failure with preserved ejection fraction in older adults. Ann Med 2012;1-14. [Epub ahead of print] PubMed Link Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA 2006;296:2209-16. PubMed Link Anand IS, Rector TS, Cleland, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE Trial. Circ Heart Fail 2011;4:569-77. PubMed Link PSAP 2013 • Cardiology/Endocrinology Connolly SJ, Camm J, Halperin JL, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011;365:2268-76. PubMed Link 13 Heart Failure with Preserved Ejection Fraction Daniel KR, Wells G, Stewart K, et al. Effect of aldosterone antagonism on exercise tolerance, Doppler diastolic function, and quality of life in older women with diastolic heart failure. Congest Heart Fail 2009;15:68-74. PubMed Link for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005;112:e154-235. PubMed Link Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:e391-479. PubMed Link Davis BR, Kostis JB, Simpson LM, et al. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation 2008;118:2259-67. PubMed Link De Boer RA, Lok DJ, Jaarsma T, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med 2011;43:60-8. PubMed Link Jacobshagen C, Belardinelli L, Hasenfuss G, et al. Ranolazine for the treatment of heart failure with preserved ejection fraction: background, aims, and design of the RALI-DHF Study. Clin Cardiol 2011;34:426-32. PubMed Link Desai AS, Lewis EF, Li R, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 2011;162:966-72.e10. PubMed Link Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:1977-2016. PubMed Link Fang Y, Debunne M, Vercauteren M, et al. Heart rate reduction induced by the current inhibitor ivabradine improves diastolic function and attenuates cardiac tissue hypoxia. J Cardiovasc Pharmacol 2012;59:260-7. PubMed Link Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory markers and incident heart failure risk in older adults. J Am Coll Cardiol 2010;55:2129-37. PubMed Link Fukuta H, Sane DC, Brucks S, et al. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation 2005;112:357-63. PubMed Link Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002;288:2144-50. PubMed Link Gaasch WH. Diagnosis and treatment of heart failure based on left ventricular systolic or diastolic dysfunction. JAMA 1994;271:1276-80. PubMed Link Kostis JB, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA 1997;278:212-6. PubMed Link Gandhi SK, Powers JC, Nomeir AM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001;344:17-22. PubMed Link Hay I, Rich J, Ferber P, et al. Role of impaired myocardial relaxation in the production of elevated left ventricular filling pressure. Am J Cardiol 2004;288:H1203-H1208. PubMed Link Krum H, Elsik M, Schneider HG, et al. Relationship of peripheral collagen markers to death and hospitalisation in patients with heart failure and preserved ejection fraction: results of the I-PRESERVE Collagen sub-study. Circ Heart Fail 2011;4:561-8. PubMed Link Heart Failure Society of America. Executive summary: HFSA 2010 comprehensive heart failure practice guidelines. J Card Fail 2010a;16:475-539. PubMed Link Little WC, Zile MR, Kitzman DW, et al. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail 2005;11:191-5. PubMed Link Hung MJ, Cherng WJ, Kuo LT, et al. Effect of verapamil in elderly patients with left ventricular diastolic dysfunction as a cause of congestive heart failure. Int J Clin Pract 2002;56:57-62. PubMed Link Maisel AS, McCord J, Nowak RM, et al. Bedside B-type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. J Am Coll Cardiol 2003;41:2010-7. PubMed Link Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heartfailure in the adult: a report of the American College of Cardiology/American Heart Association task force on practice guidelines: developed in collaboration with the American College of Chest Physicians and the International Society Heart Failure with Preserved Ejection Fraction Mak GJ, Ledwidge MT, Watson CJ, et al. Natural history of markers of collagen turnover in patients with early diastolic 14 PSAP 2013 • Cardiology/Endocrinology dysfunction and impact of eplerenone. J Am Coll Cardiol 2009;54:1674-82. PubMed Link PubMed Link Roongsritong C, Sutthiwan P, Bradley J, et al. Spironolactone improves diastolic function in the elderly. Clin Cardiol 2005;28:484-7. PubMed Link Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456-67. PubMed Link Schunkert H, Jackson B, Tang SS, et al. Distribution and functional significance of cardiac angiotensin converting enzyme in hypertrophied rat hearts. Circulation 1993;87:1328-39. PubMed Link McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441-6. PubMed Link Setaro JF, Zaret BL, Schulman DS, et al. Usefulness of verapamil for congestive heart failure associated with abnormal left ventricular diastolic filling and normal left ventricular systolic performance. Am J Cardiol 1990;66:981-6. PubMed Link McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803-69. PubMed Link Shah R, Wang Y, Foody JM. Effect of statins, angiotensin-converting enzyme inhibitors, and beta blockers on survival in patients ≥ 65 years of age with heart failure and preserved left ventricular systolic function. Am J Cardiol 2008;101:217-22. PubMed Link McMurray JJ, Carson PE, Komajda M, et al. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail 2008;10:149-56. PubMed Link Smith GL, Masoudi FA, Vaccarino V, et al. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol 2003;41:1510-8. PubMed Link Meyer P, White M, Mujib M, et al. Digoxin and reduction of heart failure hospitalization in chronic systolic and diastolic heart failure. Am J Cardiol 2008;102:1681-6. PubMed Link Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomized controlled trial. Lancet 2012 Aug 24. [Epub ahead of print] PubMed Link Mottram PM, Haluska B, Leano R, et al. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation 2004;110:558-65. PubMed Link Takimoto E, Champion HC, Li M, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 2005;11:214-22. PubMed Link Owan T, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251-9. PubMed Link Tehrani F, Morrissey R, Phan A, et al. Statin therapy in patients with diastolic heart failure. Clin Cardiol 2010;33:E1-E5. PubMed Link Packer M. Can brain natriuretic peptide be used to guide the management of patients with heart failure and a preserved ejection fraction? The wrong way to identify new treatments for a nonexistent disease. Circ Heart Fail 2011;4:538-40. PubMed Link Thohan V, Koerner MM, Pratt CM, et al. Improvements in diastolic function among patients with advanced systolic heart failure utilizing alagebrium (an oral advanced glycation end-product cross-link breaker). Circulation 2005;112(suppl 2):2647. Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539-50. PubMed Link Tribouilloy C, Rusinaru D, Mahjoub H, et al. Prognostic impact of diabetes mellitus in patients with heart failure and preserved ejection fraction: a prospective five-year study. Heart 2008;94:1450-5. PubMed Link Phan TT, Shivu GN, Abozguia K, et al. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:29-34. PubMed Link Redfield MM, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA 2003;289:194-202. PubMed Link van Veldhuisen DJ, Cohen-Solal A, Bohm M, et al. Betablockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction. J Am Coll Cardiol 2009;53:2150-8. Roger VL, Go AS, Lloyd-Jones, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 2011;123:e18-e209. PSAP 2013 • Cardiology/Endocrinology 15 Heart Failure with Preserved Ejection Fraction PubMed Link Vasan RS, Larson MG, Benjamin EJ, et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a populationbased cohort. J Am Coll Cardiol 1999;33:1948-55. PubMed Link Weber KT. Aldosterone in congestive heart failure. N Engl J Med 2001;345:1689-97. PubMed Link Westermann D, Richter RA, Jager S, et al. Enhancement of the endothelial NO synthase attenuates experimental diastolic heart failure. Basic Res Cardiol 2009;104:499-509. PubMed Link Williams ES, Shah SJ, Ali S, et al. C-reactive protein, diastolic dysfunction, and risk of heart failure in patients with coronary disease: Heart and Soul Study. Eur J Heart Fail 2008;10:63-9. PubMed Link Yamamoto K, Masayama T, Sakata Y, et al. Roles of reninangiotensin and endothelin systems in development of diastolic heart failure in hypertensive hearts. Cardiovasc Res 2000;47:274-83. PubMed Link Yip GW, Wang M, Wang T, et al. The Hong Kong diastolic heart failure study: a randomized controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 2008;94:573-80. PubMed Link Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure – abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004;350:1953-9. PubMed Link Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure. Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 2002a;105:1387-93. PubMed Link Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure. Part II: causal mechanisms and treatment. Circulation 2002b;105:1503-8. PubMed Link Zile MR, Gaasch WH, Anand IS, et al. Mode of death in patients with heart failure and a preserved ejection fraction. Circulation 2010;121:1393-405. PubMed Link Heart Failure with Preserved Ejection Fraction 16 PSAP 2013 • Cardiology/Endocrinology Self-Assessment Questions 1. A 49-year-old man has heart failure with preserved ejection fraction (HFpEF), diabetes mellitus, and hypertension. His blood pressure, which has improved during the past several months, is currently 140/75 mm Hg with a heart rate of 70 beats/minute. His home drugs include hydrochlorothiazide 25 mg/ day and metoprolol 50 mg twice daily. Which one of the following would best prevent the progression of HFpEF in this patient? C.Hypertension. D. Mitral regurgitation. 4. Which one of the following best describes the number of risk factors for HFpEF present in R.P.? A.One. B.Two. C.Three. D.Four. A.Digoxin. B.Candesartan. C.Amiodarone. D.Diltiazem. 5. Which one of the following best differentiates HFpEF from heart failure with reduced ejection fraction (HFrEF) in R.P.? A. S4 gallop. B.JVP. C. Progressive dyspnea. D.LVEF. 2. Which one of the following best describes moderate diastolic impairment on echocardiography? A. B. C. D. Left atrial pressure. Pulmonary artery pressure. Left ventricular size. E/A ratio. 6. Which one of the following is the best interpretation of R.P.’s NT-proBNP results? A. B-type natriuretic protein (BNP) would provide more utility than NT-proBNP. B. Both BNP and NT-proBNP concentrations should be assessed. C. Galectin-3 would better identify diastolic dysfunction than NT-proBNP. D. The utility of NT-proBNP may be confounded by the patient’s comorbidities. Questions 3–9 pertain to the following case. R.P. is an 80-year-old woman (height 5′5′′, weight 127 lb) being seen in the heart failure (HF) clinic. She has a 2-week history of progressive dyspnea, 1+ peripheral edema, and mild bronchiectasis. She also has a longstanding history of hypertension and recently received diagnoses of atrial fibrillation and kidney insufficiency. On physical examination, her blood pressure is 145/80 mm Hg, and her heart rate is irregular at 65 beats/minute; her jugular venous pressure (JVP) is 12 cm with a marked v wave. Auscultation detects bibasilar rales, a 2/6 systolic ejection murmur at the left sternal border, and an S4 gallop. There is also bilateral 2+ pitting edema in the lower extremities. Her echocardiography report indicates a left ventricular ejection fraction (LVEF) of 55%, biatrial enlargement, left atrial mean pressure of 19 mm Hg (normal 4–12 mm Hg), pulmonary artery pressure of 55 mm Hg (normal 15–30 mm Hg), right atrial pressure of 10 mm Hg (normal 2–6 mm Hg), and severe tricuspid regurgitation and mild mitral regurgitation. Her N-terminal pro–B-type natriuretic peptide (NT-proBNP) concentration is 240 pg/mL, and CrCL is 25 mL/minute. R.P.’s current drugs, all taken orally, include amlodipine 10 mg/ day, warfarin 4 mg/day, metoprolol 25 mg twice daily, and irbesartan 150 mg/day. 7. Which one of the following would be the most appropriate blood pressure goal for R.P.? A. B. C. D. 8. Which one of the following would be the best add-on antihypertensive therapy for R.P.? A. Furosemide 40 mg/day. B. Verapamil SR 180 mg/day. C. Hydrochlorothiazide 25 mg/day. D. Spironolactone 12.5 mg/day. 9. Which one of the following lifestyle modifications would be most appropriate for R.P.? A. B. C. D. 3. Which one of the following best describes the primary cause of R.P.’s clinical presentation? A. Atrial fibrillation. B. Left-sided filling pressures. PSAP 2013 • Cardiology/Endocrinology Less than 140/90 mm Hg. Less than 135/85 mm Hg. Less than 130/80 mm Hg. Less than 120/75 mm Hg. 17 Restrict sodium intake to less than 2–3 g/day. Reduce body mass index to less than 18. Restrict fluid intake to less than 1.5 L/day. Initiate a high-carbohydrate diet. Heart Failure with Preserved Ejection Fraction 10. Which one of the following best describes the risk of death in patients with HFpEF compared with HFrEF? D.Dronedarone. 14. E.W.’s blood pressure is currently 118/70 mm Hg. The cardiologist initiates a statin but requests your advice about the potential benefits it could provide. Which one of the following is the best reason to initiate a statin in E.W.? A. Diabetes mellitus and cerebrovascular accident increase risk of death in HFpEF versus HFrEF. B. Diabetes mellitus and cerebrovascular accident increase risk of death in HFrEF versus HFpEF. C. Diabetes mellitus, but not cerebrovascular accident, increases risk of death in HFpEF versus HFrEF. D. Cerebrovascular accident, but not diabetes mellitus, increases risk of death in HFpEF versus HFrEF. A. B. C. D. 15. A 55-year-old woman with HFpEF comes to the outpatient medicine clinic. Her blood pressure is 135/85 mm Hg, and her heart rate is 130 beats/minute. She has no other comorbidities or abnormalities. Which one of the following would be best to initiate in this patient? Questions 11–14 pertain to the following case. E.W. is a 71-year-old man (height 5′7′′, weight 300 lb) with a 1-year history of hypertension. After an aortic valve replacement for aortic stenosis, he was referred to your HF clinic with increasing dizziness during the past few days. His medical history is significant for chronic obstructive pulmonary disease (COPD). Results of his physical examination are blood pressure 89/55 mm Hg (baseline 125/87 mm Hg), heart rate 120 beats/minute, and Sao2 95% on room air. E.W. has no JVP, rales, or edema, but distant heart sounds with 1/6 systolic ejection murmur are noted. He has no other significant physical findings. Chest radiography is clear with no infiltrates. His laboratory findings include SCr 3.0 mg/dL (previously 1.0) and BUN 63 mg/ dL. E.W.’s current oral drugs include warfarin 5 mg/day, amlodipine 10 mg/day, furosemide 60 mg/day, and inhalers. His echocardiography report shows an EF of 55%. Serum lipids are TC 220 mg/dL, LDL cholesterol 135 mg/dL, HDL cholesterol 55 mg/dL, and TG 150 mg/dL. A.Enalapril. B.Metoprolol. C.Diltiazem. D.Hydrochlorothiazide. 16. You are asked by the multidisciplinary team to provide information about the role of advanced glycation end-product crosslink breakers in HFpEf. Which one of the following statements most accurately describes these novel agents? A. They act on excessive crosslinks in the myocardium but not on vasculature. B. They reduce left ventricular mass, diastolic function, and remodeling. C. They reduce intracellular calcium, leading to improved diastolic relaxation. D. They reduce pressure-induced ventricular hypertrophy and fibrosis. 11. Given E.W.’s presentation, which one of the following etiologies is most likely responsible for his HFpEF? A.Hypertension. B. Aortic stenosis. C.COPD. D.Obesity. 17. A 82-year-old woman recently received a diagnosis of HFpEF and new-onset cough. She has a history of obesity, diabetes mellitus, and hypertension. From her history, which one of the following best describes this patient’s number of modifiable risk factors? 12. Which one of the following is most appropriate for E.W. at this time? A. B. C. D. Increase his dose of inhalers. Assess his INR. Reduce amlodipine to 5 mg orally daily. Reduce furosemide dose to 20 mg/day. A.One. B.Two. C.Three. D.Four. 13. Although E.W. is no longer experiencing dizziness, he has new-onset atrial fibrillation with rapid ventricular response. Which one of the following would be best to recommend for cardioversion in this patient? 18. Which one of the following best describes the difference between the prognoses of HFrEF and HFpEF? A. The short-term mortality rate is lower with HFrEF than with HFpEF. B. The long-term mortality rate is lower with HFrEF than with HFpEF. A.Dofetilide B.Diltiazem. C.Amiodarone. Heart Failure with Preserved Ejection Fraction Improve HDL cholesterol. Improve LDL cholesterol. Increase fibrosis. Reduce afterload. 18 PSAP 2013 • Cardiology/Endocrinology C. The functional status of patients with HFrEF is similar to that of patients with HFpEF. D. Functional status declines over time with both HFpEF and HFrEF. 19. Which one of the following best describes the current evidence supporting the potential role of spironolactone in patients with HFpEF? A. Reduces fibrosis and inflammation in experimental studies. B. Improves diastolic function through antihypertensive effects. C. Improves left ventricular long-axis strain rate after 6 months of therapy. D. Reduces sudden cardiac death in symptomatic patients. 20. Which one of the following is most prevalent in both types of HF? A.Cardiomegaly. B.S3. C.JVD. D. Paroxysmal nocturnal dyspnea. PSAP 2013 • Cardiology/Endocrinology 19 Heart Failure with Preserved Ejection Fraction