Corassist - Duke Clinical Research Institute
advertisement

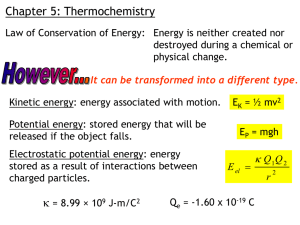
Heart Failure With Preserved EFx: Corassist Mitchell W. Krucoff MD FACC, FAHA, FSCAI Professor of Medicine / Cardiology Duke University Medical Center Director, Cardiovascular Devices Unit Duke Clinical Research Institute 20090417 Conflict of Interest Scientific Consultant, Corassist 20090417 CHF Pathophysiology Heart Failure Heart failure is the pathophysiological state in which the heart is unable to pump blood at a rate commensurate with the requirements of the metabolizing tissue or can do so only from an elevated filling pressure DHF Diastolic Heart Failure (DHF/HFPEF) Inability of left ventricle (LV) to fill properly due to stiffening and/or impaired relaxation. Increased ventricular filling pressure leads to left atrial enlargement, atrial fibrillation, pulmonary congestion SHF Systolic Heart Failure (SHF) Inability of left ventricle (LV) to contract properly due to myocardial damage leads to pulmonary and/or systemic edema 20090417 3 Therapeutic Modalities for CHF SHF: Medical: Afterload Preload Diuretics Inotropes Revascularization Mitral valve Rx Devices: ICD CRT HFPEF/DHF: Medical: Negative inotropes Surgical: Myomectomy Devices: V-pacing 20090417 15 Year Trends in CHF: Rising Incidence & Morbidity of DHF • • • • • Aging Hypertension Aortic stenosis HOCM Infiltrative Owen& Redfield MM, New England J Med. 2006;355:251-259 20090417 5 HFPEF: Animal Models ?? 20090417 Porcine (Minipig): Renal Wrap LVH/DHF Model LV Mass Increase vs. Time Shofti R., Israel Institute of Technology, The Technion, Haifa 20090417 HFPEF: Surrogate Imaging ?? 20090417 Echocardiographic surrogates: Speckle Tracking, Strain & Rotation LV rotation (Rotation and reverse rotation) LV diastolic strain and strain rate (SR E/S ratio) Norm al E A Geyer H et al, JAmSoc Echocardiogr 2010;23:351-69 Takeuchi M et al European Heart Journal (2007) 28, 2756–2762 S Carasso S J Am Soc Echocardiogr 2010;23:164-71 Carasso S et al J Am Soc Echocardiogr 2012 20090417 HFPEF: Corassist Technology 20090417 A Novel Device Construct for DHF: Systolic Spring-loading The Robin Hood Effect “Passive” energy capture from systole “Active” force for diastolic compliance & volume 2% systolic energy capture 50% increase diastolic energy delivery 20090417 HFPEF Device Platforms ImCardia® epicardial spring coils CORolla® endocardial spring *CorAssist Cardiovascular Ltd 20090417 http://www.corassist.com/index.htm ImCardia® Device Features External “pacemaker” screw anchors Cross-screw epicardial springs Standard LV postioning grid Median sternotomy implantation * *Courtesy of Renu Virmani, CV Path 20090417 Porcine Renal Wrap DHF Model 36 Week Strain Results Device - #946 Device - #788 Device - #878 Post Implantation Control - #975 1.80 Control - #206 Post Thoracotomy 1.80 1.70 1.70 Strain rate ratio 1.60 Strain rate ratio Control - #940 1.50 1.40 1.30 1.20 1.10 1.00 0.90 0.80 1.60 1.50 1.40 1.30 1.20 1.10 1.00 0.90 0.80 0 5 10 15 20 25 30 0 5 Weeks 10 15 20 25 Weeks Graph 1: Endocardial peak dia/sys strain rate ratio – Device (n=3) Graph 2: Endocardial strain dia/sys rate ratio – Control (n=3) Day 0: baseline after which renal wrapping preformed. Yellow marks – post thoracotomy Progressive deterioration in endocardial strain rate in controls (blue). Device implantation reversed strain almost to pre-renal wrapping values (magenta). 20090417 Porcine Renal Wrap DHF Model 36 Week Early Apical Untwisting Results Device - #946 Device - #878 Control - #975 Post Implantation 0.55 0.55 0.50 0.50 0.45 angle decrease [%] angle decrease [%] 0.45 0.40 0.35 0.30 0.25 0.20 0.15 0.10 0.05 0.00 Control - #940 Control - #206 Post Thoracotomy 0.40 0.35 0.30 0.25 0.20 0.15 0.10 0.05 0.00 0 5 10 15 20 25 30 0 5 10 15 20 25 Weeks Weeks Graph 1: Decrease in apical early diastolic reverse rotation – Device (n=2) Graph 2: Decrease in apical early diastolic reverse rotation – Control (n=3) Renal wrapping caused deterioration in early apical untwisting, with decreased LV suction (blue). Device implantation improved early apical untwisting almost to pre-renal wrapping values (magenta 20090417 ImCardia First In Human Single center AS patients: Clinically indicated AoVR LVH/DHF by echo N=10 unblinded implant patients N=8 concomitant observational AoVR Perioperative device tolerance demonstrated (safety) 24 month follow up in 14/18 patients 20090417 ImCardia® 24 month AoV Gradient & EFx Aortic Peak Gradient Ejection Fraction 20090417 17 ImCardia® 24 month LA Area, LV Mass LA Area LV Mass 20090417 18 CORolla Technology Platform Endocardial nitinol spring Increased energy transfer efficiency Trans-apical insertion (thoracotomy) Retractable http://www.corassist.com/demo_corolla.htm 20090417 Trans-Apical Sheep Deployment: Hadassah Ein-Kerem Animal Laboratories, Jerusalem 20090417 20 CORolla ® Pre Clinical – In Vivo Results Results and conclusions to date: • No procedure-related or device-related deaths • Animal recuperation was quick • No embolic events or endocardial erosion 20090417 21 Device Therapy for Diastolic HF (DHF): Conclusions HF with preserved systolic function is a large and rapidly growing source of morbidity & health care costs Surgical, medical and device therapies for DHF have limited therapeutic impact Animal models of DHF are very limited Systolic energy “loading” of metallic diastolic spring technology represents a novel device-based approach to DHF FIM using surgically implanted epicardial springs in a human AS model appears safe and possibly effective Trans-apical delivery of an intraventricular spring coil may augment both diastolic efficiency and ease of use 20090417 Heart Failure With Preserved EFx: Corassist Mitchell W. Krucoff MD FACC, FAHA, FSCAI Professor of Medicine / Cardiology Duke University Medical Center Director, Cardiovascular Devices Unit Duke Clinical Research Institute 20090417