Lab #1 Supplement: Scientific notation logarithms, pH, and metric
advertisement

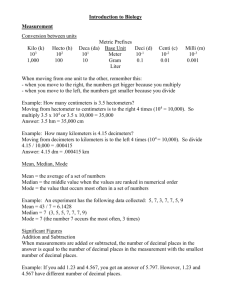
Lab #1 Supplement: Scientific notation logarithms, pH, and metric conversions 1. A quick refresher on scientific notation: Scientific notation is a convenient way of expressing very large or very small numbers. In this method, numbers are expressed as a root value multiplied by some factor of 10 (10, 100, 0.1, etc). To use this method effectively, though, you must be comfortable with exponents and be able to switch from scientific notation to decimal notation and vice versa when needed. Numbers expressed as scientific notation are presented as a single-digit value (followed by any fractional values) multiplied by some factor of 10 (for example, 6.25 × 1010). There should be one and only one digit to the left of the decimal (for example, 62.5 × 109 is incorrect notation, as is 0.925 x 10-2). You can think of the exponent in scientific notation as indicating the number of digits that separate the first non-zero number from the decimal point. For example: 1 × 102 = 100.0 Note that there are two zeros separating “1” from the decimal point. Here are some other examples 1 × 100 = 1 1 × 101 = 10 5 × 102 = 500 6.1 × 104 = 61,000 If the exponent is negative, it indicates the number of digits the first non-zero number is positioned to the right of the decimal point. 1 × 10-1 = 0.1 5 × 10-2 = 0.05 7 × 10-3 = 0.007 3.4 × 10-4 = 0.00034 You can also try a technique of moving the decimal point. For large numbers (10 or greater), move the decimal point over to the left until there is only a single non-zero digit to the left of the decimal point. The number of digits you had to move the decimal point to the left is equal to the exponent. For example, we might convert 256,000,000 into scientific notation in the following manner: 256000000.0 = 2.56 × 108 8 7 6 5 4 3 2 1 For numbers less than 1.0, move the decimal point to the right until one non-zero digit is to the left of the decimal point. Each digit you moved the decimal point to the right is equal to -1 in the exponent. For example, 0.000000292 could be converted into scientific notation as such: 0.000000292 = 2.92 × 10-7 1 2 3 4 5 6 7 Give it a try on your own. If you want to use a calculator to check your answers, often scientific calculators have an ‘EE’ (or ‘EXP’) button that will allow you to enter in numbers in scientific notation (EE = “times 10 to the power”, so don’t multiply the value by 10 first then hit EE). Enter in the nonzero values, then press EE and enter the exponent. To do the opposite (convert scientific notation into decimal form), you would simply use the opposite method—move the decimal point to the right for the number of digits equal to the exponent if the exponent is positive, or to the left if the exponent is negative. Sample problems: (Answers are provided at the end of this sheet). Express the following in scientific notation (i.e. × 10x) 1. 0.004 2. 126,900 3. 0.00000001 4. 50 5. 0.167 Express the following in decimal form 6. 6 × 104 7. 1.35 × 10-5 8. 1 × 101 9. 1 × 10-7 10. 6.02 × 1023 2. Logartihms and Calculation of pH Remember, pH is an index of hydrogen ion concentration in solution. It is calculated in the following manner: pH = log(1/[H+]). Logarithms scare students at times, but they are actually quite easy to understand once you are comfortable with scientific notation. Basically, a logarithm (specifically, a base-10 logarithm) is the exponent by which you would multiply 10 by itself in order to get a particular value. For example: 100 = 102 and log 100 = 2 1000 = 103 and log 1000 = 3 Notice that in these examples the logarithm’s first non-zero value is equal to the exponent of the value expressed in scientific notation. Effectively, a logarithm’s first digit indicate the number of digits the value is positioned to the left or right of the zero. Here are a couple of parallel number lines to demonstrate: Decimal 0.01 0.1 1 10 100 Scientific 1×10-2 1×10-1 1×100 1×101 1×102 Logarithm -2 -1 0 1 2 The logarithm can therefore indicate the size of a value in factors of 10. For example, a value between 10 and 100 will have a logarithm value between 1 and 2 (e.g. log 50 = 1.69897), and a value between 100 and 1000 will have a logarithm value between 2 and 3 (e.g. log 450 = 2.65321). Logarithms of numbers between 1 and 0 will be negative, and the first number will indicate the number of digits to the right of the decimal point before the non-zero numbers begin. For example, values between 0.1 and 1 will have a logarithm between 0 and –1 (e.g. log 0.3 = -0.523), and values between 0.01 and 0.1 will have a logarithm between –2 and –1 (e.g. log 0.07 = -1.1549). With that under your belt, calculating pH should be a snap. Try these examples: Sample Problems: (Answers are provided at the end of this sheet). Calculate the pH of the following solutions given their [H+] 11. 1 × 10-9 12. 3 × 10-14 13. 5 × 10-5 14. 4 × 10-2 15. 1 3. Metric Conversions Throughout the semester we will be expressing quantities using metric values1, which are the standard method of measurement throughout the world (and, since they are all based on each other and are expressed by factors of ten, are much more useful and frankly make a lot more sense than the English measurement system now used only in the United States and to a lesser degree in Great Britain). The most common measurements you will see in this course are measurements of mass (grams), volume (liters), and linear distance (meters). The metric system has a standardized series of prefixes that are used to express different multiples of a particular measurement (see Table 1.1), which is useful for describing these measurements on different scales. For example, using the English system, we probably would not say that distance between Kokomo and Indianapolis is ~264,000 feet. Rather, we would express this distance as ~50 miles (50 multiples of 5,280 feet). Likewise, we would not say that the width of your hand is ~0.0000631 miles, but would rather express that width as ~4 inches. The metric system’s prefixes are used to describe quantities at different scales based on factors of 10 (which are much easier to convert from one unit to another than by factors of 12 or 5,280 or the like). However, this does require some familiarity with the various prefixes. Moreover, you should be able to make some conversions from multiples at one scale to those of another. For example, rather than expressing a volume as 0.005 liters, it would be more appropriate to describe it as 5 milliliters (five one-thousandths of a liter). Table 1.2 (next page) provides common conversions for multiples used in this course. Table 1.1. Common prefixes used to express multiples of metric units. Metric prefix Tera-(T) Giga-(G) Mega-(M) kilo-(k) -----deci-(d) centi-(c) milli-(m) micro-(µ) nano-(n) pico-(p) Value Trillion Billion Million Thousand ----Tenth Hundredth Thousandth Millionth Billionth Trillionth Decimal 1,000,000,000,000 1,000,000,000 1,000,000 1,000 1 0.1 0.01 0.001 0.000001 0.000000001 0.000000000001 Scientific Notation 12 × 10 9 × 10 6 × 10 3 × 10 0 × 10 -1 × 10 -2 × 10 -3 × 10 -6 × 10 -9 × 10 -12 × 10 Table 1.2. Conversions of some common multiples of metric values for mass, volume, and distance. Mass kg = 1000g 1,000,000 mg 1,000,000,000 µg g = 0.001 kg 1000 mg 1,000,000 µg Volume L = 1000 ml 1,000,000 µl ml = 0.001 L 1000 µl µl = Distance m = 100 cm 1000 mm 1,000,000 µm cm = 0.01 m 10 mm 10,000 µm 0.000001 L 0.001 ml mg = 0.000001 kg 0.001g 1000 µg mm = 0.001 m 0.1 cm 1000 µm µg = 0.000000001 kg 0.000001 g 0.001 mg µm = 0.000001 m 0.0001 cm 0.001 mm Answer Key to Sample Problems: 1. 4 x 10-3 2. 1.269 x 105 3. 1 x 10-8 4. 5 x 101 5. 1.67 x 10-1 6. 60,000 7. 0.0000135 8. 10 9. 0.0000001 10. 602,000,000,000,000,000,000,000 11. 9 12. 13.523 13. 4.301 14. 1.398 15. 0