REVISED MATERIAL TO EXPLAIN WHY 1,2
advertisement

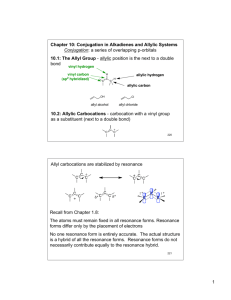
REVISED MATERIAL TO EXPLAIN WHY 1,2-ADDITION OF ELECTROPHILES TO CONJUGATED DIENES OCCURS FASTER THAN 1,4-ADDITION. [The explanation on p. 311 of your textbook is also found in most (but not all) introductory organic texts. A brilliantly conceived experiment by the late Eric Nordlander and his students at Case Western Reserve University makes it clear that this explanation based on "attack of the nucleophile at the site of greater positive charge" cannot be correct. What Nordlander did (as will be explained by Paula Bruice in greater detail below) was to examine a symmetrical allylic cation in which positive charge is equally distributed to its two termini. (Of course he had to destroy the symmetry in order to differentiate between the 1,2 and 1,4 products, but he did this with the minimal perturbation of one deuterium.) The result was that 1,3-pentadiene reacted with D-Cl, at low temperature, to give about a 75/25 mixture of 1,2/1,4 products in media ranging from neat to pentane to acetic acid to nitromethane. Clearly, a fully-formed symmetrical allylic cation (with chloride ion positioned equally distant from C2 and C4) is not produced here. He explained the 1,2 preference by noting that deuteronation at C1 generates a chloride ion near C2; covalent bonding to C2 occurs faster than a symmetrical ion-pair can be formed. The details, they can be found at J. Am. Chem. Soc. 1979, 101, 1288. If this explanation is valid, then it would seem that it can (and should) be used for the parent case of 1,3-butadiene + H-X.] ************************************************************************************************ Prof. Bruice has written the following material for her fourth edition. The first two paragraphs replace the paragraph beginning "The reactant for the second step ..." on p. 311. The material below PROBLEM 11 replaces much of the next-to-last paragraph on p. 313. The words are Prof. Bruice's; I merely provided the drawing to aid your understanding. NEW AND REVISED Now we need to see why the 1,2-addition product is formed faster. In other words, why is the transition state for its formation more stable than the transition state for formation of the 1,4-addition product? For many years chemists thought it was because the transition state for formation of the 1,2-addition product resembles the contributing resonance structure in which the positive charge is on a secondary allylic carbon, whereas the transition state for formation of the 1,4-addition product resembles the contributing resonance structure in which the positive charge is on a primary allylic carbon. However, when the reaction of 1,3-pentadiene + DCl was carried out under kinetic control, essentially the same relative amounts of 1,2- and 1,4-addition products were obtained as were obtained from the kinetically controlled reaction of 1,3-butadiene + HBr. The transition states for formation of the 1,2- and 1,4-addition products from 1,3pentadiene should both have the same stability because both would resemble a contributing resonance structure in which the positive charge is on a secondary allylic carbon. Why then is the 1,2-addition product still formed faster? Step (1) Cl Cl H D H2C H 4 2 3 1 H + D H2C CH3 5 3 1 at C2 Cl ca. 75% H H2C 1 4 2 3 H 5 H2C + 4 2 1 3 CH3 5 H 4 2 3 1 CH3 1,2-addition 5 H + H2C CH3 H H Cl H D H D H Step (2) H 4 2 H D H CH3 5 at C4 ca. 25% H D H2C 1 Cl H 4 2 3 CH3 1,4-addition 5 H When the B electrons of the diene abstract D+ from a molecule of undissociated DCl, the chloride ion can stabilize the positive charge at C-2 better than the positive charge at C-4 simply because, when the chloride ion is first produced, it is closer to C-2 than to C-4. So it is a proximity effect that causes the 1,2-addition product to be formed faster. A proximity effect is an effect caused by one species being close to another species. PROBLEM 10 a. Why does deuterium add to C-1 rather than to C-4 in the above reaction? b. Why was DCl rather than HCl used in the above reaction? PROBLEM 11 a. When HBr adds to a conjugated diene, what is the rate-determining step? b. When HBr adds to a conjugated diene, what is the product-determining step? Because the greater proximity of the nucleophile to C-2 contributes to the faster rate of formation of the 1,2-addition product, the 1,2-addition product is the kinetic product for essentially all conjugated dienes. But do not assume that the 1,4-addition product is always the thermodynamic product. The structure of the conjugated diene is what ultimately determines which is the thermodynamic product. For example, the 1,2addition product is the thermodynamic product in the case of the reaction of 4-methyl1,3-pentadiene with an electrophilic reagent such as HBr, and the 1,2- and 1,4-addition products obtained from the reaction of 2,4-hexadiene with HCl have the same stability – they both have the same number of alkyl groups bonded to their carbons. Thus neither product is themodynamically controlled. New Problems for the end of the chapter 1. The experiment shown below and discussed in Section 7.8 showed that the proximity of the chloride ion to C-2 in the transition state caused the 1,2-addition product to be formed faster than the 1,4-addition product. a. Why was important for the investigators to know that the reaction was being carried out under kinetic control? b. How could the investigators determine that the reaction was being carried out under kinetic control? 2. A student wanted to determine if the greater proximity of the nucleophile to the C-2 carbon in the transition state was what caused the 1,2-addition product to be formed faster when 1,3-butadiene reacts with HCl. Therefore, he decided to investigate the reaction of 2-methyl-1,3-cyclohexadiene with HCl. His friend, Noel Noall, told him that he should use 1-methyl-1,3-cyclohexadiene instead. Should he follow his friend’s advice?