A molecular orbital (MO) energy level diagram - Parkway C-2
advertisement
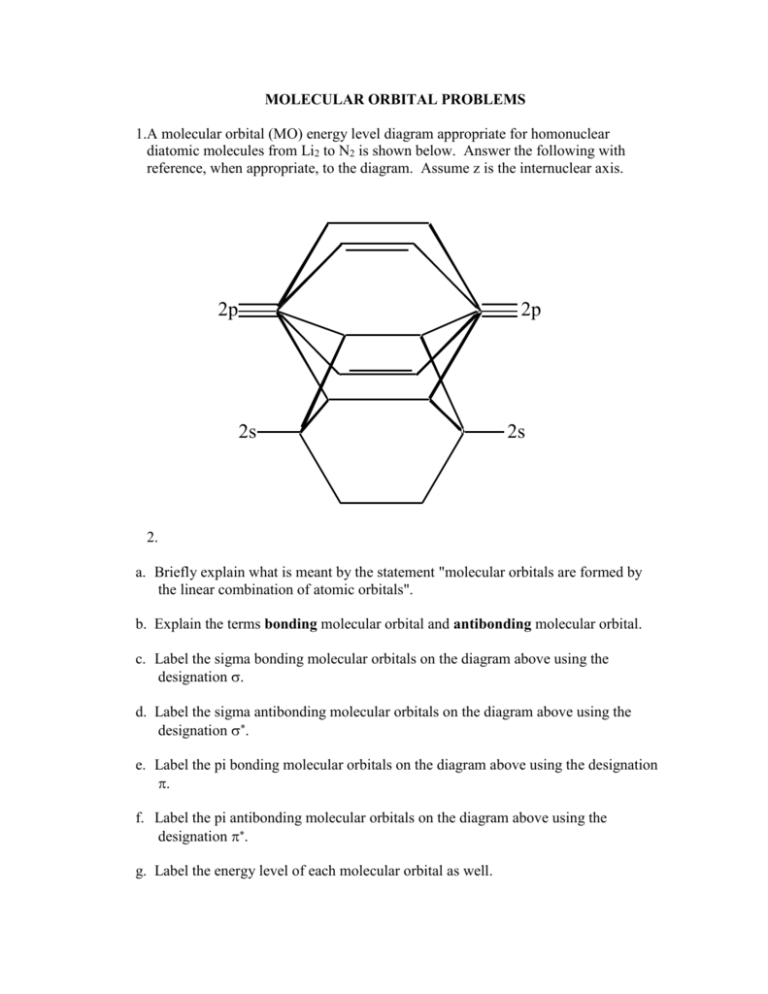
MOLECULAR ORBITAL PROBLEMS 1.A molecular orbital (MO) energy level diagram appropriate for homonuclear diatomic molecules from Li2 to N2 is shown below. Answer the following with reference, when appropriate, to the diagram. Assume z is the internuclear axis. 2p 2p 2s 2s 2. a. Briefly explain what is meant by the statement "molecular orbitals are formed by the linear combination of atomic orbitals". b. Explain the terms bonding molecular orbital and antibonding molecular orbital. c. Label the sigma bonding molecular orbitals on the diagram above using the designation . d. Label the sigma antibonding molecular orbitals on the diagram above using the designation . e. Label the pi bonding molecular orbitals on the diagram above using the designation . f. Label the pi antibonding molecular orbitals on the diagram above using the designation . g. Label the energy level of each molecular orbital as well. 3. a. The MO energy level diagram does not show the 1s orbitals. Explain. b. Explain why the 2p orbitals are shown to be involved in the formation of the 2s* orbital. Which atomic orbitals contribute most to the 2s* orbital? c. Briefly comment on why the 2p orbital energy is higher than the 2p orbital energy for these molecules. 4. a. Give the electron configurations for the species C2 and C22-. (C: Z = 6) b. State, with explanation, whether each species is diamagnetic or paramagnetic. c. How is bond order calculated? d. Give the bond orders of C2 and C22-. e. Give the relative bond lengths and bond strengths of C2 and C22- meaning, compare them to one another. Which is more stable? f. BN (boron nitride) is isoelectronic with C2 (i.e. they have the same number of electrons). Briefly explain how the MO diagram shown on the previous page would have to be modified to represent the bonding in BN. 5. What is the bond order of the listed bonds in the following molecules or ions? a. CO in carbonate ion e. OO in oxygen in O2 b. CO in acetic acid f. CN in hydrogen cyanide c. NO in nitrite ion g. SF in sulfur tetrafluoride d. CC acetylene h. CO in carbon dioxide 6. Using the same ordering of the orbitals as for homonuclear diatomic molecules from Li2 to N2 as shown above, draw the MO diagram for the cyanide anion, CN-, for the cyanide radical, CN, and for the cation CN+. Answer the following for each of the three: a) How many unpaired electrons are there? b) Is it paramagnetic or paramagnetic? c) What is the bond order? 7. A molecular orbital (MO) energy level diagram appropriate for homonuclear diatomic molecules from O2 to F2 is shown below. a. Using the above molecular orbital diagram, illustrate the electron configuration for O2. b. How many unpaired electrons are there? c. Is it paramagnetic or dimagnetic? Draw the classic Lewis dot diagram for O2. Does this suggest that O2 is paramagnetic or diamagnetic? d. What is the bond order, based on your MO diagram? e. Using a MO diagram, illustrate the electron configuration for O2+. Is it more or less stable than O2? Why? 8. Illustrate the classic Lewis dot diagram for N2. a. Should it be paramagnetic or diamagnetic? Why? b. Using an appropriate molecular orbital diagram, illustrate the electron configuration for N2. c. Experimental data suggests that liquid nitrogen is not magnetic. Is this consistent with localized electron (LE) theory, or molecular orbital (MO) theory? Why? 9. Using a MO diagram, illustrate the electron configuration for CO, carbon monoxide. Assume that it follows the MO diagram appropriate for homonuclear diatomic molecules from Li2 to N2. a. What is the bond order for CO? b. Does the bond order suggest that CO bonds are stable or unstable? Is this consistent with LE theory? c. Why do we assume that the valence orbitals in oxygen are at a lower energy than those of carbon?










