Grade 10 Science – Unit 2
advertisement

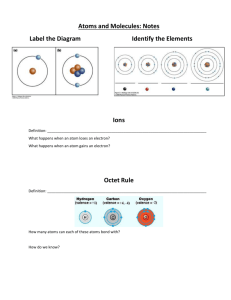
Grade 10 Academic Science - Chemistry Elemental Notation Each atom has an atomic number and atomic mass. The atomic number represents the number of protons in the nucleus AND it is the atomic number that determines the identity of the element. The atomic mass number represents the total number of protons plus the number neutrons in an atoms nucleus. As shown, the carbon atom has an atomic number of 6 and an atomic mass number of 12. Thus, carbon has six protons and six neutrons. Protons have a positive charge, electrons are negative and neutrons have no charge. Since the atom has an overall neutral charge, the number of protons must equal the number of electrons. Task – Complete the following table using the Periodic Table in your textbook. Elemental Notation Sr Number of Protons 3 Number of Electrons 3 Number of Neutrons 4 Br Na Ne O Ba Grade 10 Academic Science –Chemistry Lewis Dot Diagrams Lewis Dot Diagrams are very useful. With them, you can (1) determine the type(s) of covalent bonds that an element may make, and (2) predict the type of ion that an atom might make. Each dot diagram consists of an elemental symbol (called the kernel) and a group of 1-8 dots which shows the configuration of the outer-most electron shell of the atom (i.e., the valence shell). This is an example of the proper Lewis dot diagram for the element oxygen. The "O" represents the kernel (the nucleus and all of the electrons except those in the valance shell). Each of the four "sides" of the symbol represents an orbital in the outermost energy level of the atom. Since each orbital can hold only two electrons, the sides of the dot diagram can only hold up to two dots. The six dots show the configuration of the valence electrons. To make a Lewis Dot Diagram Step 1 Step 2 . . X X . You need to know how many electrons are in the valence shell. You fill in one valence electron on each side of the elemental symbol, and then double up as many sides as you need to in order to include each one. Each side can only hold up to two dots. By looking at the Lewis Dot Diagram for oxygen, you can see that oxygen has two unpaired electrons, so it has two electrons available for standard bonds. These unpaired electrons might make two single covalent bonds (e.g., water (H2O)) or they might make one double covalent bond, as the case of magnesium oxide (MgO). When Lewis dot diagrams are used to illustrate compounds, "x's" are often used to substitute for the dots of one or more elements to show which electrons came from which element. For example, the Lewis Dot Diagrams for both oxygen and hydrogen as free elements, and then, water as a compound. Task – Draw the Electron Configuration and Lewis Dot Diagram for the following elements Magnesium Electron Configuration Lewis Dot Diagram Aluminum Electron Configuration Lewis Dot Diagram Phosphorus Electron Configuration Lewis Dot Diagram Argon Electron Configuration Lewis Dot Diagram Boron Electron Configuration Lewis Dot Diagram Carbon Electron Configuration Lewis Dot Diagram Neon Electron Configuration Lewis Dot Diagram Nitrogen Electron Configuration Lewis Dot Diagram Sodium Electron Configuration Lewis Dot Diagram Fluorine Electron Configuration Lewis Dot Diagram