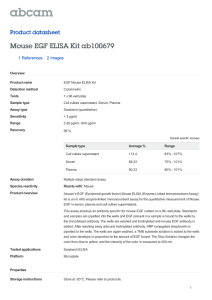
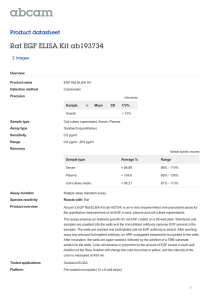
Adhesion assay protocol – Rada Vukmirovic 10/22/00

Adhesion assay protocol – Rada Vukmirovic
Materials:
24 well plates-Falcon (3847), A surface
=20mm, V well
=3.5ml
Fn-
BSA-
EGF-
Media:
Cell Growth Media
Serum free Media
10/22/00
Assay Media
Assay Procedure:
1.
Coat the 20mm surface with Fn: 0.5 ml of appropriate concentration of Fn (0.1,
0.3, 1, 3
g/ml) in PBS
2.
Incubate for 2 hours
3.
Aspirate the left over Fn and rinse the well twice with PBS
4.
Block non-specific protein adhesion in 1% BSA (of PBS) for 1 hour.
5.
Aspirate and rinse with PBS plates twice
6.
Store at 4 o
C
Adhesion Assay:
1.
Plate 24-well plates with 20,000 cells per well in serum free media for 12 hours in the 5% CO
2
incubator .
2.
Change media to assay media with or without 25nM EGF
3.
Short term adhesion with EGF was measured after 30 min. after the addition of
EGF
4.
Adhesion was also measured after incubation for 8 hours in the air incubator
5.
Fill the wells with media and seal using sealing tape. Avoid air bubbles.
6.
Invert plates and fasten plates on the centrifugation clamps.
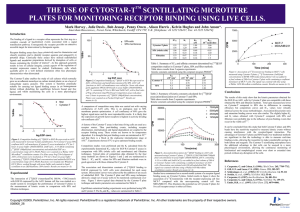
7.
Spun the plates in the swing bucket SH-3000 rotor in a bench-top Sorvall centrifuge for 10 min at 25 o
C at 400, 600, 800 x g. During each experiment one plate at 3
g/ml Fn coating concentration without EGF was kept at 1g and used as a control. Cell number was quantified by manually counting cells in a defined well area. At least four wells were used at each condition and four fields were counted per well with each field in the control containing 300-400 cells. The cell number was normalized to the average cell number in the control well to obtain the fraction adherent cells.
8.
The amount of centrifugal force required to detach 50% of the cells (F
50
) was obtained from a plot of fraction adherent cells versus centrifugal force.
9.
The mean detachment force was calculated using the equation
f = RCF*V* (
c
-
m
), where f is a force on the cell, RCF is the relative centrufugal force, v is the cell volume,
c is the density of the cell, and
m
is the density of the medium.