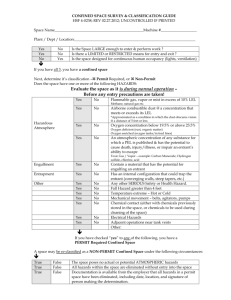
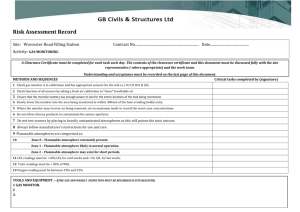
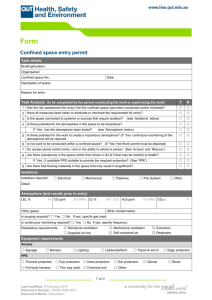
Confined Space Rescue
advertisement

Confined Space Rescue Training Topics I II III IV VI Respiratory Protection Anatomy and Physiology Confined Space Emergencies Toxic Atmosphere Monitoring Equipment Breathing Apparatus Review Lifting Systems Training Continued: VII Confined Space Rescue Practical Exercises References NFPA 1670 Operations and Training for Technical Rescue Incidents NFPA 1006 Professional Qualifications for Rescue Technicians Confined Space and Structural Rope Rescue, Michael Roop/Tom Vines/Richard Wright. Mosby Press 1997 References OSHA 29 CFR 1910.146 Compliance Directive for Permit Required Confined Spaces Technical Rescue Field Operations Guide, Tom Pendley. Desert Rescue Research 2000 Respiratory Protection The Respiratory Process The exchange of gases (O2 & CO2) between the alveoli & the blood occurs by simple diffusion: O2 diffusing from the alveoli into the blood & CO2 from the blood into the alveoli. Respiratory Process Cont. We do this, of course, by breathing - continuously bringing fresh air (with lots of O2 & little CO2) into the lungs & the alveoli. Breathing is an active process - requiring the contraction of skeletal muscles. The primary muscles of respiration include the external intercostal muscles (located between the ribs) and the diaphragm (a sheet of muscle located between the thoracic & abdominal cavities). The Respiratory Process Confined Space Emergencies Confined Space Fatalities: – 90 % due to asphyxiation – 60 % of the fatalities are would be rescuers Ex.-1990: 3 Firefighters die in Pennsylvania from Co poisoning from running portable pump Example: PA Deaths On May 1, 1990, a 39-year-old male volunteer firefighter died inside a 33-foot-deep water well in Pennsylvania while attempting to pump water out of the well. Also, two male volunteer firefighters (ages 40 and 20) died attempting rescue. http://www.cdc.gov/niosh/injury/traumacsface.html#1990 (other examples) C-Space Definition OSHA 29 CFR 1910.146 – An OSHA confined space is defined as: A. B. C.Examples Examples include but are not limited to: Permit Required C-Space A confined space permit is required if the space has one or more of the following hazards: – 1. – 2. – 3. – 4. Non-Permit C-Space A non-permit required confined space is: – 1. Spaces that do not contain, – 2. Space in which all the hazards C-Space Entry Risk Profile A permit required confined space has less risk if it meets the following criteria: – A. The internal configuration of the space is Entry Risk Pro-file cont. – B. The victim can be easily – C. Rescuers can pass easily through – D. The space can accommodate – E. All hazards in and around the space C-Space Entry Risk Profile A permit required confined space has more risk if any of the following conditions or other hazardous conditions exist – A. – B. – C.Entry Risk Profile cont. – D. (Interpreted from NFPA 1670) C-Space Hazards It should always be considered that the most unfavorable situation exists in every confined space and that the danger of explosion, poisoning, and asphyxiation will be present at the onset of the emergency Hazard Types Hazards specific to a confined space are dictated by: 1.- – Ex. Damp activated carbon in a filtration tank will absorb oxygen, creating an oxygen deficient atmosphere Hazard Types cont: 2-: – Such as the fermentation of molasses that creates ethyl alcohol vapors and decrease the oxygen content of the atmosphere Hazard Types cont: 3– As in the case of sewer systems that may be affected by rising water, heavier than air gases, or flash floods The most hazardous kind of confined space is the type that combines limited access and mechanical devices C-Space Hazard Groups Confined space hazards can be grouped into the following categories: – 1. – 2. – 3. – 4. Oxygen Deficient Atmosphere Normal atmosphere composed of % oxygen, % nitrogen and % argon An atmosphere containing less than % oxygen shall be considered oxygen deficient *O2 levels inside confined spaces may be decreased as the result of consumption or displacement* Effects of decreasing O2 Levels Level of 17 % – Between 14-16 % – Between 6-10 % – Less than 6 % – Consumption of O2 Takes place during combustion of flammable substances During bacterial action During chemical reactions as in the formation of rust Displacement of O2 Gas that displaces oxygen and therefore reduce the O2 levels Nitrogen, argon, helium and carbon dioxide are used as inerting agents to displace flammable substances and retard pyrophoric reactions O2 Enriched Environment An atmosphere containing more than % of oxygen is oxygen enriched and enhances the flammability of combustibles Flammable materials such as clothing and hair burn violently when ignited Flammable Atmospheres Arise from enriched O2 atmospheres, vaporization of flammable liquids, byproducts of work, chemical reactions or concentrations of combustible dust Work conducted in a c-space can generate flammable atmospheres Flammable Atmosphere Terms ________________________is the lowest temperature at which a liquid can form an ignitable mixture in air near the surface of the liquid. The lower the flash point, the easier it is to ignite the material (at the flash point, the flame does not need to be sustained). Example Gasoline has a flash point of -50 degrees F (-45 C) and is more flammable than ethylene glycol (antifreeze) which has a flash point of 111 degrees C (232 F) Flammable Atmosphere Terms _____________________ the temperature at which the flame becomes self-sustained so as to continue burning the liquid The fire point is usually a few degrees _____________________ the flash point Flammable Atmosphere Terms ____________________________ apply generally to vapors and are defined as the concentration range in which a flammable substance can produce a fire or explosion when an ignition source (such as a spark or open flame) is present The concentration is generally expressed as percent fuel by volume UEL/LEL _____________________________ (UFL) the mixture of substance and air is too rich in fuel (deficient in oxygen) to burn. This is sometimes called the upper explosive limit (UEL) _____________________________ (LFL) the mixture of substance and air lacks sufficient fuel (substance) to burn. This is sometimes called the lower explosive limit (LEL) Example UEL/LEL It is usually quite easy to reach the lower flammable limit. There are numerous cases where individuals have used a solvent, sealer, or other flammable materials in a basement or closed room with inadequate ventilation...and have been injured when the vapors were ignited by a pilot light, electric spark or other ignition source Example UEL/LEL Newcastle in September of 2003 – A pipe fitter left an acetylene cylinder inside his vehicle over the weekend. Either the cylinder had a small leak or the valve was not fully closed. The flammable limits for acetylene are extremely broad, _____% to ____% in air – When the worker opened the door, an undetermined spark source (the door light switch, light bulb, cellular phone, static etc.) ignited the mixture with catastrophic results Acetylene Explosion Flammable Atmosphere Terms ________________________________ (PEL) is the maximum amount or concentration of a chemical that a worker may be exposed to under OSHA Regulations _________________________________ (TWA) - are an average value of exposure over the course of an 8 hour work shift Flammable Atmosphere Terms _____________________________________ (IDLH) atmospheres poses an immediate threat to life, would cause, irreversible adverse health effects, or would impair an individual's ability to escape from a dangerous atmosphere Flammable Atmospheres Flammable gases such as or vapors from hydrocarbons can be trapped in c-spaces than air will seek lower levels as in pits, sewers, storage tanks/vessels Gases Flammable Atmospheres In a closed top tank, lighter than air gases may rise and develop a flammable concentration if trapped the opening Combustible dust concentrations are found during loading/offloading, conveying grain products, nitrated fertilizers and finely ground chemical products Toxic Atmospheres The source of toxic atmospheres encountered in c-spaces may arise from: – – – Toxic Atmospheres Carbon Monoxide – Odorless, colorless gas, approximately the same density of air – Formed from incomplete combustion of organic materials – Can be formed from mircobial decomposition of organic materials in sewers/silos and fermentation tanks Measuring Toxicity Measured in terms of permissible exposure limit (PEL) PEL is the concentration of a toxin that most people could safely be exposed to for an 8 hour period Any toxin in a confined space greater than its PEL is hazardous Irritant (Corrosive) Atmospheres Irritant gases vary widely among all areas of industrial activity They can be found in plastic plants, chemical plants, petroleum industry, tanneries, refrigeration industries, paint manufacturing and mining operations Irritant (Corrosive) Atmospheres Prolonged exposure at irritant or corrosive concentrations in a c-space may produce little or no evidence of irritation Danger in this situation is that worker is usually not aware of any toxic exposure Examples: nitrogen dioxide, sulfur dioxide, ammonia Mechanical/Physical Hazards Vibrations/moving machinery – Augers, hydraulics, steam, etc. Noise – Noise problems intensified in c-space because interior causes sound to reverberate – May disrupt verbal communication with emergency personnel on the exterior of the space Toxic Atmosphere Monitoring Equipment Atmospheric monitoring should take place continuously or at frequent intervals during the rescue operation All atmospheric monitoring equipment should meet OSHA standards Equipment should be calibrated according to manufacturer’s recommendations Atmospheric Testing Procedures First set of tests should be performed by remote probe prior to entering the space All levels of the space need to be metered due to (weight of a vapor compared to air) Principles of Air Monitoring meters to manufacturer’s spec If O2 level is not normal, readings will be affected Spaces may have stratified atmospheres, levels of space must be metered Allow for air intake in sampling hose/probe, approx. _________ ppm = ______ % sec per of hose Meters Oxygen Levels According to OSHA, air containing less than 19.5 % or more than 23 % oxygen is unacceptable If oxygen level is not normal, flammability readings will be effected Atmosphere Flammability Measured in the % of the lower explosive limit (LEL) The LEL is the lowest concentration of a product that will explode or burn when it contacts a source of ignition of sufficient temperature OSHA -> C-space is hazardous if it contains more than ______ % of the LEL Lower Explosive Limit LEL A flammable gas must reach 100 % of its LEL to ignite and burn Meters are usually calibrated with a flammable gas such as methane, heptane or pentane Lower Explosive Limit LEL Methane LEL -> approximately 5 % Different gases have different LELs Meter calibrated to methane will give an inaccurate reading for a gas with a different LEL Meter reading of 10 % or less of the LEL should ensure that an atmosphere is below the LEL of most gases Common Gas Examples Methane (CH4): – – – LEL %, UEL % Nitrogen (N2): – – – Common Gases Carbon monoxide (Co): – – – PEL = – TWA = – LEL ____%, UEL ____ % – IDLH =Common Gases Hydrogen Sulfide (H2S): – – – Odor thresh hold = – – LEL = ____ %, UEL = ____ % – Hydrogen Sulfide Cont: – PEL = – TWA = – IDLH = ppm Toxic Atmospheres Known materials: -Use meter specific to that chemical to test for these products Unknown materials: -Use meters to take readings and narrow the spectrum of chemicals -Broad spectrum analysis -Colormetric tubes Hazard Abatement Hazard Reduction Reducing or abating hazards of a confined space emergency is essential before entry is safe In addition to protective equipment, SCBA, other measures should be taken externally OSHA requires that measures be taken before permit spaces are entered Electrical Usually isolated by a combination of: – 1. – 2. Hydraulic Includes liquids, finely divided solids that if not secured may cause exposure or engulfment Usually isolated by: – 1. – 2. Mechanical Hazards in the space or introduced into the space Includes energy from: – – Ventilation Why Ventilate?? When atmospheric conditions is a c-space do not meet the limits for O2, flammability and toxic vapors, the c-space must be ventilated to bring the atmosphere into those limits. Methods of Ventilation 1. 2. 3. Positive Pressure (Supply) Direction of fresh air flow into space creating a positive pressure diluting any contaminants by the addition of fresh air _______________________ operated fans should be used to prevent unacceptable levels of Co into space by use of gasoline blowers Air flow should be introduced into the space and the flow should be at the level at which rescuers will be working Positive Pressure Fan should be allowed to operate long enough to exchange the air content of the space several times Capacity of fan in cubic feet per minute (CFM) divided into the volume of the space in cubic feet = the time it takes to exchange air one time Positive Pressure (Supply) Positive pressure (supply) can force air into space ________times the distance exhaust (negative) pressure can draw it Examples Super Vac's AirPac 25 duct canister allows the 25 ft. x 8 in. duct to be easily stored and rapidly deployed Negative Pressure (Exhaust) Exhausts contaminants from the space (using negative pressure) by pulling contaminated air out of a space A slight vacuum is created that can draw other contaminants into the space May draw flammable gases over motor Positive-negative/push-pull Flushes the atmosphere by supplying and exhausting large volumes of air Two portals must be present, positive air flow into space while negative pressure pulls contaminants out Most ________________ method for ventilation Consider where the contaminated exhaust is going and if it will pose an additional hazard Respiratory Protection Types of SCBA OSHA CFR 1910 direct that unless the cause of the emergency can be established as NOT atmosphere related, fresh air breathing apparatus must be worn Types: – Self contained breathing apparatus (SCBA) – Supplied air respirator (SAR) Self Contained Breathing Apparatus Positive pressure since 1983 Prevents contamination of the air inside the face piece if a leak occurs in the face piece’s seal Limited amount of air supply (based on wearers personal characteristics) Supplied Air Respirators During C-space rescue, conventional SCBA’s size often makes it difficult to use SCBA small enough to pass through narrow openings may limit duration of its air supply to impractical levels Supplied Air Respirators are a viable option SAR Components SAR consists of: – Open circuit face piece – Regulator – Egress cylinder attached via a low-pressure air line to remote source air supply (restricted to maximum distance allowed by manufacturers, usually no more than 300 feet from point of attachment) SAR Components OSHA requires an SAR used in an atmosphere that is immediately dangerous to life and health (IDLH) have an additional supply Must be capable of providing enough air for the wearer to escape the atmosphere in the event the primary supply is interrupted SAR Components “Escape” requirement addressed by attaching small breathing air cylinder rated at 5 minutes to the SAR unit 5 minute cylinder are intended to provide enough air for escape although they may be incapable of doing so SAR Air Carts Survivair Air Cart Contains up to two independently operated 30-,45-, or 60-minute high pressure (4500 psi) cylinders Or to two independently operated 30 minute low pressure (2216 psi) cylinders An optional accessory case can hold a variety of Hip-Pac and hose combinations Survivair Air Cart Two inlets allow regulated or unregulated external air sources to be used Built-in manifold has four Foster or Schrader quick-disconnect couplings to supply air for up to four workers Used in any confined space where an SCBA would reduce or restrict worker movement OSHA Respiratory Standard 1910.134(e)(3)(iii) requires, when an IDLH atmosphere exists, A stand by man or men with suitable self contained breathing apparatus shall be at the nearest fresh air base for emergency rescue Safe Respiratory Work Practices 1. Rescuers should immediately 2. Rescuers should wear 3. Minimum capacity of of the source air should be ____________ the volume of the total needs of all rescuers connected to it for the anticipated duration of the rescuer’s entry Safe Work Practices cont: 4. A minimum team of __________ rescuers should be utilized for all permit space rescue entries Lifting/Raising Systems Miller Tripod Miller Tripods provide a highly portable anchorage system for typical confined space entry and rescue systems Made of high-strength aluminum, the tripod withstands up to 5,000 lbs of pull yet weights only 42 lbs Legs lock independently and adjust with integral push pins allowing set up on uneven surfaces SKED EVAC Tripod Features aircraft-grade, gold-anodized aluminum legs and a cast-aluminum head Three heavy-duty rigging anchors have exceptionally large holes for easy attachment and are located in the center SKED EVAC Tripod Legs adjust in 5-inch increments for a maximum height of 10 feet and a minimum length for transport of 7 feet Holes in the feet allow the tripod to be bolted into position 119 inch height / 5,280 lbs (23kN) SKED EVAC Tripod Mechanical Advantage Systems Retrieval Systems 1910.146 (k)(3) requires that retrieval systems be used except when the retrieval equipment would increase the risk to an entrant or would not contribute to the rescue of an entrant. When a retrieval system is not used, alternate methods of retrieval must be developed. MA Systems Rescuer hauler 4:1 system – 3-inch double pulley with a cam – rope can move in only one direction when the cam is engaged – allows rescuer to raise a load by pulling on the tail end of the rope, releasing it, and getting another grip MA Systems Rescuer hauler 4:1 – cam can be released manually by pulling on the attached cord – accommodates rope sizes from 3/8” (10mm) to 1/2” (12/5mm). – Minimum break strength when in use is 12,000 lb Patient Evacuation Devices Patient packaging devices that can be used in confined spaces include but are not limited to the following: – – – – – – – – Prefabricated Class III Ha C-Space Rescue Priority 1: Make the scene safe – – Priority 2: Victim contact by Primary Rescuer – – – – C-Space Rescue Priority 3: Size-up Priority 4: Preparation - C-Space Rescue Priority 5: Access Victim Priority 6: Stabilize and package victim C-Space Rescue Priority 7: Evacuate Priority 8: Response Termination Rescue Response Non-IDLH Atmosphere – Incident Commander – Rescue Sector Officer – Entry Supervisor: Verifies Determines that Removes Terminates entry Rescue Response – Attendant: Knows Knows Remains Communicates Monitors Calls Prevents Performs no Rescue Response – Entrant (Primary): Knows Recognizes Recognizes Uses proper Communicates with Alerts Rescue Response – Entrant (Stand-by): Knows Recognizes exposure Recognizes effects of Uses proper Communicates with Alerts attendants of Rescuer for primary Rescue Response – Support Personnel: – Safety Officer: Oversees scene In matters of safety, has over the incident commander During rescue, each rescuer should consider him/herself equally responsible for safety IDLH Atmosphere – Incident Commander – Rescue Sector Officer – Entry Supervisor: Verifies tests required are complete Determines that space remains safe during work Removes unauthorized persons from space area Terminates entry if conditions are poor/degrading IDLH Atmosphere – Attendant: Knows space hazards Knows effects of exposure Remains outside space at all times Communicates with entrant(s) Monitors entry activities Calls RESCUE if needed Prevents unauthorized entry Performs no conflicting duties IDLH Atmosphere – Entrant (Primary # 1): Knows space hazards Recognizes exposure signs/symptoms Recognizes effects of exposure Uses proper PPE Communicates with attendant Alerts attendants of hazards IDLH Atmosphere – Entrant (Primary # 2): Knows space hazards Recognizes exposure signs/symptoms Recognizes effects of exposure Uses proper PPE Communicates with attendant Alerts attendants of hazards IDLH Atmosphere – Entrant (Stand-by # 1): Knows space hazards Recognizes exposure signs/symptoms Recognizes effects of exposure Uses proper PPE Communicates with attendant Alerts attendants of hazards Rescuer for primary entrant IDLH Atmosphere – Entrant (Stand-by # 2): Knows space hazards Recognizes exposure signs/symptoms Recognizes effects of exposure Uses proper PPE Communicates with attendant Alerts attendants of hazards Rescuer for primary entrant IDLH Atmosphere – Support Personnel: Ventilation/metering/air watch/decon, etc. – Safety Officer: Oversees scene for safety hazards In matters of safety, has authority over the incident commander During rescue, each rescuer should consider him/herself equally responsible for safety