Hydroxymethylene - Metro State Atheists
advertisement

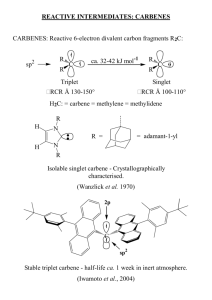
Chalmer Wren December 2, 2008 Advanced Organic Chemistry Introduction Carbenes are a family of organic molecules composed of a neutral divalent carbon atom with a sextet of electrons.2 The two primary categories that carbenes can be divided into are singlets and triplets. Singlet carbenes are extremely reactive and, until recently, their existence was thought too transitory to be directly observed.2 In fact, carbenes were not even commercially available until 1995. In 1954, Doering and Hoffman established dichlorocarbene as an intermediate in the hydrolysis of chloroform under basic conditions, thereby introducing carbenes into organic chemistry.3 In 1964, carbenes were introduced into organmetallic chemistry by Fischer.3 Recently, in 2008, the existence of the simplest carbene, methlyene, was observed directly using spectroscopy.1 Numerous examples of carbenes can be seen in Table on page 7. Spectroscopy Due to the instability inherent to carbenes, studying them with spectroscopy, as in general, is challenging. Time resolved absorption spectroscopy and matrix isolation spectroscopy have been the most potent and rewarding means of studying carbenes.8 The former of these means of study allows the for the observation of reaction kinetics, and has allowed determination of carbene lifetimes and absolute reaction rates.8 In matrix isolation spectroscopy, a carbene is trapped in a solid matrix at extremely low temperatures. By performing the process at low temperatures, thereby minimizing thermal energy, the carbene product is kinetically stabilized. Generally, a precursor to the photochemical production of carbene, which are frequently diazo compounds such as V-12 from Figure 12, is situated in the matrix and photochemically converted to the carbene product.8 The matrix then immobilizes the carbene. Two kinds of organic matrices can be used; organic glass or rare gas matrices. For an organic glass, the photochemical precursor to a carbene is mixed with an organic solvent and then frozen, forming an organic glass matrix. However, the high reactivity of carbenes limits the use of all but the most inert organic solvents and, in for the more reactive carbenes, organic glass matrices can’t be used at all. In such cases, the use of a rare gas matrix is necessary.8 Developed by Whittle et al., a mixture of the carbene precursor and an inert gas are sprayed onto a spectroscopic window at sufficiently cold temperature to cause immediate condensation and formation of the matrix.9 Methylene was observed using this method, with argon as the matrix.1 Diazomethane can also be reacted with acyl chloride, I-12, to produce a carbene.7 The reaction, depicted in Figure 12, begins with acylation, wherein the lone pair of the carbon in diazomethane forms a bond to the electron deficient carbon of the carbonyl group, replacing the chlorine and forming the diazonium compound II-12. Then, a second diazomethane molecule acts as a base, taking a proton from the –carbon of II12. This results in the formation of III-12, a diazocarbonyl compound. Applying heat or light causes III-12 to decompose into the nitrogen gas and the carbene IV-12. The diazocarbonyl is an example of a photochemical precursor that could be used to produce a carbene in matrix isolation spectroscopy. Matrix isolation spectroscopy not only allows for characterization of carbenes, but also serves as tool for investigating bimolecular reactions of carbenes. This is accomplished by doping the matrix with some secondary reactant. After matrix formation and carbene immobilization, the matrix can be heated to release the carbene which subsequently reacts with the secondary reactant used to dope the matrix.8 The products of this reaction can then be spectroscopically studied and characterized. Carbene Structure Carbenes can be sp-hybridized or sp2-hybridized. Carbenes that are sp-hybridized have a linear geometry while sp2-hybridized carbenes have a bent geometry. An sp2hybridized carbene has a nonbonding orbital and a nonbonding py orbital, hereafter designated as the p orbital for an sp2-hybridized carbene. For an sp2-hibridized carbene with bent geometry, the four possible electronic configurations are depicted in Figure 1. Figure 1 A B C D Didier Bourissou, Olivier Guerret, François P. Gabbaï, and Guy Bertrand. Chem. Rev., December 22, 1999.100 (1), 39 –92. Figure 1A depicts a triplet state with pconfiguration 3B1 statewherein the two nonbonding electrons are in different orbitals, one in the p orbital and one in the s orbital, while have parallel spins. Figure 1B depicts a singlet state with configuration 1A1 statewherein the two-nonbonding electrons are both in the orbital. Figure 1C depicts a singlet state with pconfiguration 1A1 statewherein the two-nonbonding electrons are both in the porbital. Figure 1D depicts an excited singlet state with p configuration (1B1 statewherein the two nonbonding electrons are in different orbitals while having anti-parallel spins.3 Carbenes with a linear configuration are sp-hybridized, having two nonbonding degenerate orbitals px and py, and are usually triplet carbenes. With the exception of dihalogenocarbenes and carbenes with sulfur, nitrogen, or oxygen bond directly the to divalent carbene, which likely exist as singlets, most carbenes are non-linear triplets.10 The preferred singlet state for dihalogenocarbenes and carbenes with nitrogen, oxygen, or sulfur substituents can be explained by resonance stabilization, depicted in Figure 4 and Figure 7. Evidence Of Existence In 1954, Doering and Hoffman established dichlorocarbene as an intermediate in the hydrolysis of chloroform under basic conditions.5 They reacted cyclohexene with chloroform in the presence of the strong base potassium t-butoxide.5 In the mechanism they proposed t-butoxide first removes the proton from chloroform, resulting in the formation of t-butyl alcohol and the conjugate base of chloroform. The conjugate base of chloroform, a trichloromethide anion, forms chloride ion and a carbene known as dichlorocarbene. The mechanism for the formation of dichlorocarbene is shown in Figure 2. Figure 2 Cl Cl Cl H + O - Cl - + HO Cl Cl chloroform Cl C t-butoxide trichloromethide ion t-butyl alcohol C Cl dichlorocarbene Then, as shown in Figure 3, dichlorocarbene goes on to react with cyclohexene, forming 1,1-dichloro-2,2-dimethylcyclopropane in what is referred to as a cycloaddition. The reaction is likely a concerted process. Figure 3 Cl C Cl Cl + Cl dichlorocarbene cyclohexene 1,1-dichloro-2,2-dimethychyclopropane Doering and Hoffman did not observe dichlorocarbene directly but, rather, trapped the carbene as a stable adduct and then deduced its existence as the best possible explanation for the observed results. Cycloaddition reactions can occur for both triplet and singlet carbenes.10 As discussed in Spectroscopy, such carbenes have since been observed directly. Carbene Reactivity Because singlet usually carbenes have an unoccupied orbital and an orbital occupied by a lone pair of electrons, some should display ambiphilic character.3 Ambiphilic species are those that are stabilized by both electron-donating and electronwithdrawing species and therefore have the potential to react with both electrophilic and nucleophilic species.4 The unoccupied orbital gives the carbene carbon its electrophilic character, while the lone pair imparts the carbene carbon with the potential for nucleophilic character. The ground state multiplicity of a carbene is influenced by inductive, mesomeric, and steric effects.4 Inductive Effects The electronegativity of a carbene substituent influences whether the ground state is a triplet or singlet state. -electron withdrawing substituents increase the s character of the occupied nonbonding orbital, thereby inductively stabilizing the carbene in the singlet state depicted in Figure 1B.3 electron donating substituents favor the triplet state by destabilizing nonbonding orbital.3 Mesomeric Effects Another primary contributor to the structure and stability of a carbene is the mesomeric effect, which is also known as the resonance effect. The stability of a carbene substituent Substituents that donate electron density are designated with an X, while substituents that withdrawal electron density are designated with a Z. (X,X)-Carbenes, i.e., carbenes for which both substituents donate electron density, should be sp2hybridized singlets.3 Donating electron density to the empty p orbital increases its energy, thereby increasing the magnitude of energy required for an electron to move from the orbital to the p orbital, thereby favoring the carbene configuration depicted in Figure 1B.3 Through resonance, the p orbital and the adjacent p orbitals the substituents interact to form partial double bond character. The resonance structures of (X,X)-carbene is depicted in Figure 4. Figure 4 X C X + X C X X C X+ (Z,Z)-Carbenes, i.e., carbenes whose substituents withdrawal electron density, should be sp-hybridized singlet carbenes.3 The unoccupied substituent p orbitals interact mesomerically with the py orbital to form a 2-electron 3-center system .3 1999, (Z,Z)carbenes have not been isolated .3 The resonance structures of a (Z,Z)-carbene is depicted in Figure 5. Figure 5 Z C Z + Z C + Z Z C Z (X,Z)-Carbenes are neither linear or nonlinear, but instead are classified as quasilinear. The X substituent lone pair interacts with the py orbital, while the Z substituent interacts with the px orbital .3 The singlet state for (X,Z)-carbenes is favored .3 Bulky substituents kinetically stabilize all types of carbenes, and steric effects may even dictate the ground state multiplicity if mesomeric and inductive effects are negligible .3 Carbene Synthesis In addition the reaction of chloroform with strong base, discussed earlier and shown in Figure 2, several other means of producing carbenes exist. For example, the carbene know as methylene can be produced by subjecting diazomethane to photolysis, which is depicted in Figure 6.7 Figure 6 H - C + N N hv N N H + C H H Synthesis of Stable Singlet (X,X)-Carbenes Aminocarbenes The stability of singlet carbenes is largely dictated by substituents associated with it. For example, the stability of carbenes can be increased using amino substituents. This is because the adjacent nitrogen atoms decrease the electron deficiency of p orbital by donating electron density through resonance, while stabilizing the lone pair of the orbital of carbon by inductively withdrawing electron density.3 The resonance of diamniocarbene is shown in Figure 7. + N N C N N C C N + N Figure 7 Realizing the potential for amino groups to stabilize carbenes, Wanzlick attempted to synthesize the aminocarbene species II, depicted in Figure 8, in 1962.3 This was accomplished the thermal elimination of chloroform.3 Only the product, III, was isolated and subsequent cross-coupling experiments failed to verify the existence of an equilibrium between III and the carbene intermediate.3 Figure 8 Ph Ph N N Cl N Ph Ph Cl N N C Cl N Ph Ph Ph Ph II I N N III Despite Wanzlick’s failure, the isolation of stable aminocarbenes was eventually achieved. In 1990, Arduengo, Harlow, and Kline reported the synthesis, structure, and characterization of the first stable crystalline carbene. The crystalline carbene they isolated was 1,3-di-1-adamantylimidazol-2-ylidene. They produced the carbene at room temperature by deprotonating 1,3-di-1-adamantylimidazolium chloride with sodium hydroxide in tetrahydrofuran. Dimsyl anion was used as a catalyst. The same carbene can be produced using potassium t-butoxide. 1,3-di-1-adamantylimidazol-2-ylidene is a clear colorless crystalline species and is stable in the absence of moisture and oxygen.6 In 1995 it was reported that the carbene known as 1,2,4-triazol-5-ylidene, designated as I-9 in Figure 9, could be produced from a corresponding 5-methoxy-1,3,4triphenyl-4,5-dihydro-1H-1,2,4-triazole, designated as II-9 in Figure 9, in quantitatively useful yields, making it the first commercially available carbene. The process, depicted in Figure 9, was accomplished via thermal elimination of methanol at 80C and 0.1 millibars.3 Additional examples of amino stabilized carbenesinclude II, III, IV, V from Table 1. Figure 9 Ph N Ph N N Ph I-9 Ph O Ph N C N N Ph II-9 + OH Table 1 Didier Bourissou, Olivier Guerret, François P. Gabbaï, and Guy Bertrand. Chem. Rev., December 22, 1999.100 (1), 39 –92. Figure 10 H3C H O C - + C N N Cl Cl H - H O + C C N N H3C I-12 - C C CH3 V-12 H - H heat or uv N O C C C + N NH III-12 H + O CH3 II-12 N + C N N H H O H C CH3 IV-12 + N N Citations 1) Phantom Parent Molecule Of Important Class Of Chemical Compounds Isolated For First Time. Science Daily [Online] June 17, 2008. http://www.sciencedaily.com/releases/2008/06/080611135106.htmhttp://www.scienc edaily.com/releases/2008/06/080611135106.htm (accessed November 1, 2008). 2) Peter R. Schreiner, Hans Peter Reisenauer, Frank C. Pickard IV, Andrew C. Simmonett, Wesley D. Allen, Mátyus & Attila G. Császár. Nature. June 12, 2008. 453, 906-909. 3) Bourissou D., Guerret O., Gabbaï F., and Bertrand G. Chem. Rev., December 22, 1999.100 (1), 39 –92. 4) By Samir Z. Zard. Oxford University Press. 2003. pp 11. http://books.google.com/books?id=BspMJ-hN_pgC&pg=RA1-PA4IA7&lpg=RA1-PA4IA7&dq=what+does+ambiphilic+mean&source=web&ots=tdUKcXaTMz&sig=u RdUfYxS7rM0qC5CgvatXatkj_U. (accessed November 1, 2008). 5) W. von E. Doering, A. Kentaro Hoffman. J. Am. Chem. Soc. 1954, 76, 6162. 6). Anthony J. Arduengo III. Richard L. Harlow, Michael Kline. J. Am. Chem. Soc. 1995, 117, 11027. 7) Nick Greeves, Alex Lawrenson, Kirsty Barnes. (2007) University of Liverpool. ChemTube3D – Interactive 3D Organic Reaction Mechanism. Carbenes. http://osxs.ch.liv.ac.uk/~ng/external/CarbenesPhotolysisDiazomethaneCarbene.html (accessed November 9, 2008). 8) http://0scitation.aip.org.skyline.cudenver.edu/getpdf/servlet/GetPDFServlet?filetype=pdf &id=JCPSA6000022000011001943000001&idtype=cvips (accessed November 11, 2008). 9) Whittle, E.; Dows, D.A.; Pimentel, G.C. J. Chem. Phys. 1954, 22, 1943. 10) Gilchrist, T. L.; Rees C.W. (1969) Appleton-Century-Crofts. p. 1-8