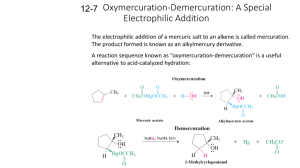
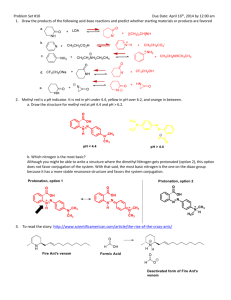
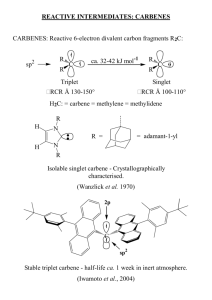
lit_jacs128_45_14676_06
advertisement

Literature Discussion Spring 2007 The following refers to: J. Am. Chem. Soc., 128 (45), 14676 -14684, 2006. 1. What are the authors trying to accomplish here? In your answer, include: a) What is a carbene? What is the difference between a Schrock and Fischer carbene in structure, binding, and reactivity? b) What types of catalysis are carbenes used for (it might help to look up the references under footnote 2)? Describe in detail a typical mechanism for carbene catalysis. c) Why are transition metal carbenes much more common than alkali or alkaline earth carbenes? d) How are transition metal carbenes typically synthesized? How are alkaline earth metal carbene complexes synthesized? How are main group metal (i.e. Sn or Pb) carbene complexes synthesized? How was the title compound synthesized in this paper? e) Fundamentally, why is this new compound interesting and exciting to the authors (and to all inorganic chemists!)? 2. Consider the related complex H2CCa: a) The bonding in this complex can be broken down into two parts: the bonding of the H’s to the C and then the bonding of the :CH2 to the Ca. Derive the two SALCS of the H2 fragment, showing clearly how you determine their symmetries. Determine the symmetries of the valence orbitals of the C under the appropriate symmetry. Construct an MO diagram for the formation of [:CH2] from the M and the H2-fragment, showing which MOs are occupied. Do the best you can estimating the orbital energies. The end result should look something like the MO diagram for H2O. Then, take these orbitals and interact them appropriately with Ca (first, determine the symmetries of the valence 3d, 4s, and 4p orbitals of the Ca under the appropriate symmetry then interact them with the appropriate symmetries of the :CH2 fragment). When you finish, you will have the MO diagram for H2CCa. b) Using symmetry and point group tables, how many vibrations will be IR active? Raman active? c) Determine the expected bond order for H2CCa. 3. Trace the Ca-C, Ca-N and C-P bond lengths from 4-H2 (the ligand), (4-H)2Ca (the monodeprotonated ligand-Ca complex), to (4-Ca)2 the carbene dimer. Are the trends in the experimentally determined bond lengths the same as the calculated ones? What do the changes in bond lengths tell you about the bonding both within the ligand and the ligand to Ca? How are the Ca-C and Ca-N bond lengths related? Why? You might want to think about the carbene as a possible π acid and what this might do to bond lengths. 4. Why do you think complex [4-Ca]2 forms as a dimer and not a monomer? What is the 13C NMR shift for the C in (4-Ca)2? How does this compare with transition metal carbenes? Why? Why are the analogous Li, Cr, and Pb dimmers NMR silent? (Hint: look at the numbers of unpaired electrons). Describe what happened by NMR when the investigators dissolved their complex in THF. Draw the likely resulting product. Do you think the mechanism for the ligand exchange is likely associative or dissassociative? Why? Does the complex’s reactivity with THF tell you anything about the binding of the ligand to the Ca? 5. Discuss the bonding of the carbene to Ca. Drawing on your discussion in question 3, what would you expect the charge distribution on each atom to be? What did the authors find when they did the calculations on a simplified model? Discuss the conclusions you can draw from the results in Table 2 and figure 4 (calculations on the original complexes) and relate them back to what you expected the charges to show. It is not enough here to simply list what the authors have concluded. You need to put these conclusions in your own words and show how the authors got there and why it is important. Was there any difference between calculations on the simplified and non-simplified model? How do the calculations of partial charges help you understand the geometric features of the molecules? 6. Discuss the initial reactivity studies the authors have completed on [4-Ca]2 (Scheme 3). What general trends in reactivity do you see? How might you expect the reactivity of this compound to be different than the reactivity of a transition metal analogue? Propose one additional reaction (NOT discussed in the paper) that you would like to see the authors perform. What do you expect as a product (and why)? 7. Take a look at the crystal structures summarized in Figure 1-2, and the corresponding experimentals. Why were these crystal structure determinations done at low temperature? What was the R factor of each of the structures? What does this mean? Some of the hyrdrogens were “found” and refined, the others were estimated. Why is “finding” hydrogens difficult by X-ray diffraction?