Supplementary Information (doc 2631K)

Supplementary Information
DNA-based Advanced Logic Circuits for Nonarithmetic Information
Processing
Hailong Li,
1,2,3,4
Yaqing Liu,
1,2
Shaojun Dong
1,2
* and Erkang Wang
1,2
*
1
State Key Lab of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry,
Chinese Academy of Sciences, Changchun 130022, Jilin, China
2
Graduate School of the Chinese Academy of Sciences, Beijing 100039, China
3
Department of Nanomedicine, Houston Methodist Research Institute, Houston, Texas 77030,
United States
4
Department of Cell and Developmental Biology, Weill Medical College of Cornell University,
New York 10065
* E-mail: dongsj@ciac.jl.cn; ekwang@ciac.jl.cn
1.
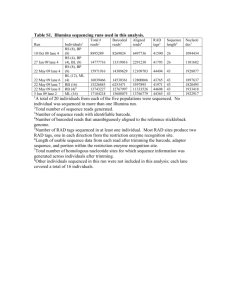
Multiplexer: DNA sequences design.
The detailed sequences of associated DNA strands in constructing the 2:1 multiplexer.
Learned from Figure 1C , the presence of MUX-IN 1 leads to formation of MB-MUX-IN
1 complex through hybridization of Segments I and II in MUX-IN 1 to MB (hybridization and the two-base mismatch is detailed in Figure S1 ), opening MB and thus producing highly fluorescent FAM (curve b in Figure 1D ). However, neither MUX-IN 2 nor MUX-IN 3 (Address
input: A) hybridizes with MB. Therefore, only weak FAM fluorescence was observed upon applying either input to the MB platform (curve c and e in Figure 1D ). In the simultaneous presence of two inputs, MUX-IN 1 and MUX-IN 2, the interaction between MUX-IN 1 and MB is designed to be superior to any other DNA hybridizations, resulting in MB-MUX-IN 1 complex and leaving MUX-IN 2 alone. A strong FAM fluorescence signal can be read due to the separation of FAM from Dabcyl quencher in the formed MB-MUX-IN 1 complex (curve d in
Figure 1D ). In the coexistence of MUX-IN 1 and MUX-IN 3 inputs, the interaction between the inputs is designed to dominate the reactions in the system, producing MUX-IN 1-3 complex through consecutive hybridization of Segments II and III in MUX-IN 1 to Segments II, I and V in MUX-IN 3 ( Figure S2 ). The MB platform stays alone accompanied by weak FAM fluorescence (curve f in Figure 1D ). In the last case of simultaneous two inputs, MUX-IN 2 and
MUX-IN 3, a three-component complex is expected to be formed through hybridization among the two inputs and the MB platform. In brief, Segments I, IV and III in MUX-IN 2 are fully complementary to Segment I, V, and IV in MUX-IN 3 to generate MUX-IN 2-3 complex ( Figure
S3 ), the unpaired segments of which are able to hybridize with MB, finally leading to formation of MB-MUX-IN 2-3 three-component complex as well as strong FAM fluorescence signal (curve g in Figure 1D ). Upon simultaneous presence of all the three inputs, MB-MUX-IN 1 and MUX-
IN 2-3 complexes are the main products in the system, which is also accompanied by a strong
FAM fluorescence signal due to MB opening in MB-MUX-IN 1 complex (curve h in Figure
1D ). The interaction among all DNA strands associated with the present 2:1 multiplexer was further confirmed by PAGE analysis (see Figure S4 and related discussion there).
2.
Demultiplexer: DNA sequences design and discussion of associated results.
The detailed sequences of associated DNA strands in constructing the 1:2 demultiplexer.
Learned from Figure 2C , DEMUX-IN 1 is capable of opening MB to generate high FAM fluorescence through hybridization between Segment I in DEMUX-IN 1 and MB, which is confirmed by the strong FAM fluorescence response (curve b Figure 2D ). DEMUX-2 is
designed not to hybridize with MB, and thus the resultant FAM fluorescence signal is weak
(curve c in Figure 2D ). Besides, no G-quadruplex can be produced in the presence of DEMUX-
IN 1 or DEMUX-IN 2, generating a low NMM fluorescence (curve b and c in Figure 2E ). In the coexistence of both inputs, DEMUX-IN 1 prefers to hybridize with DEMUX-IN 2 to form
DEMUX-IN 1-2 complex rather than with MB to form MB-DEMUX-IN 1. The reason is that the hybridization in DEMUX-IN 1-2 complex through fully complementary Segments I and II in both of them is much more stable than that in MB-DEMUX-IN 1 complex. Therefore, a weak
FAM signal is monitored in this case (curve d in Figure 2D ). In addition, the hybridization between DEMUX-IN 1 and DEMUX-IN 2 favours the formation of G-quadruplex via G-rich
Segments III in both of them, producing highly fluorescent G-4/NMM complex (curve d in
Figure 2E ). The G-quadruplex formation in turn further increase the interaction between the two inputs. The interactions among all the DNA strands associated with the developed 1:2 demultiplexer was further confirmed by PAGE analysis (see Figure S5 and related discussion there).
3.
Encoder: DNA sequences design and the interactions among them.
Sequences of DNA inputs used in the operation of the 4-to-2 encoder.
Learned from Figure 3B , Neither strong FAM fluorescence nor strong NMM fluorescence was observed in the presence of EC-IN 0 alone (curves b in respective Figure 3C and 3D ), which is equal to that of the initial state without any inputs (curves a in respective
Figure 3C and 3D ). The hybridization between EC-IN 1 and MB results in the opening of MB, and thus only highly fluorescent FAM is generated (curves c in respective Figure 3C and 3D ).
Input EC-IN 2 does not interact with MB, where only highly fluorescent G-4/NMM complex is formed (curves d in respective Figure 3C and 3D ). Finally, the input EC-IN 3 to the MB platform leads to MB-EC-IN 3 complex due to hybridization between Segment I in EC-IN 3 and
MB, accompanied by a strong FAM fluorescence signal as well as a strong NMM fluorescence
signal according to the G-rich Segment II in EC-IN 3 (curves e in respective Figure 3C and 3D ).
The interaction among associated DNA strands is further confirmed by PAGE analysis (see
Figure S6 and related discussion there).
4.
Design of decoder and discussion of associated results.
Sequences of DNA inputs used in the operation of the 2-to-4 decoder.
Learned from Figure 4B , in the absence of any inputs, only a strong FAM signal was observed in Output 0 channel (curve a in Figure 4C ), while the signals in the other output channels were weak, including NMM, HEX, and pyrene-based excimer fluorescence (curves a in
Figure 4D , 4E and 4F , respectively). Upon input DC-IN 0, the interaction between MB and
MBC will be disturbed because partial segment I in DC-IN 0 is designed to be fully complementary to MBC. DC-IN 0 would replace MB strand in MB-MBC complex to form much more stable duplex. As a result, MB platform is re-formed, and thus the FAM fluorescence intensity is weakened (curve b in Figure 4C ). Meanwhile, G-quadruplex is formed from the Grich Segment II in DC-IN 0, producing a strong NMM fluorescence signal in Output 1 channel
(curve b in Figure 4D ). In this condition, both HEX signal in Output 2 and Excimer signal in
Output 3 are weak (curves b in Figure 4E and 4F , respectively). When DC-IN 2 is present alone, its partial Segment II is also fully complementary to MBC, leading to recovery of MB platform and thus weak FAM fluorescence (curve c in Figure 4C ). In this case, HEX fluorescence in
Output 2 channel is strong (curve c in Figure 4E ) while weak NMM and Excimer fluorescence are monitored in the other output channels (curves c in Figure 4D and 4F , respectively). At last, the simultaneous presence of both inputs results in two kinds of complexes. One is DC-IN 0-1 complex, which favors the formation of highly fluorescent excimer in Output 3 channel from attached pyrene in each input strand (curve d in Figure 4F ). The other is a four-component complex formed from DC-IN 0, DC-IN 1 and two MBC, resulting in the re-formation of MB
molecular beacon and the corresponding weak FAM fluorescence (curve d in Figure 4C ).
Besides, in either complex, the G-rich Segment II in DC-IN 0 to form fluorescent G-4/NMM complex in combination with NMM is also inhibited through hybridization to Segment I in DC-
IN 1 (curve d in Figure 4D ), which also brings HEX in proximity to G-rich Segment III in DC-
IN 0, resulting in weak HEX fluorescence due to the quenching ability of G base (curve d in
Figure 4E ). It should be noted that the G-rich plus (Segment III in DC-IN 0) does not significantly affect Segment II to form G-4/NMM complex ( Figure S9 ). The interaction among associated DNA strands are further confirmed by PAGE analysis (see Figure S10 and related discussion there).
Figure S1. Hybridization between MB and MUX-IN 1. The two-base mismatch can be clearly seen.
Figure S2. Hybridization between MUX-IN 1 and MUX-IN 3.
Figure S3.
Hybridization between MUX-IN 2 and MUX-IN 3.
Figure S4.
Native polyacrylamide gel analysis of the interactions among MB, MUX-IN 1,
MUX-IN 2, and MUX-IN 3 (Address input: A) DNA strands. MUX-IN 1, MUX-IN 2, and
MUX-IN 3 is abbreviated as M1, M2, and M3, respectively. The sample in each lane and the identities of the main belts are indicated above and at the sides of the gel image, respectively.
Discussion of Figure S4 :
PAGE analysis of the interactions among the four DNA strands (MB and three inputs) in the 2:1 multiplexer is achieved through belt comparison. The belts in lane 1, 2, 3, and 4 correspond to MB, MUX-IN 1, MUX-IN 2, and MUX-IN 3 (Address input: A), respectively.
The weak visibility of these belts may be ascribed to their respective diffusion. The new belts in lane 6, 7, and 8 indicate the formation of MUX-IN 1-3, MUX-IN 2-3, and MB MUX-IN 1 complexes, respectively. On contrast, MUX-IN 1 and MUX-IN 2 does not interact with each other (lane 5) through comparison with lane 2 and 3, and so is MB and MUX-IN 2 in lane 9 as well as MB and MUX-IN 3 in lane 11. In lane 10, the mixture of MB, MUX-IN 1 and MUX-IN
2 generates a clear belt ascribed to MB MUX-IN 1 complex and weakly visible MUX-IN 2 belt.
In lane 12, the mixture of MB, MUX-IN 1 and MUX-IN 3 produces two clear belts, which belong to MB and MUX-IN 1-3 complex, respectively, through comparison with lane 1 and 6. In lane 13, the clear belt is ascribed to three-component MB MUX-IN 2-3 complex in comparison with lane 1 and 7. In lane 14, two clear belts corresponding to MB MUX-IN 1 and MUX-IN 2-3 complexes appear in the presence of all three inputs.
Figure S5.
Native polyacrylamide gel analysis of the interaction among MB, DEMUX-IN 1, and DEMUX-IN 2 (Address input: A) DNA strands. DEMUX-IN 1 and DEMUX-IN 2 are abbreviated as DM1 and DM2, respectively. The sample in each lane and the identities of the main belts are indicated above and at the sides of the gel image, respectively.
Discussion of Figure S5 :
PAGE analysis of the interaction among the three DNA strands (MB and two inputs) in the
1:2 demultiplexer is achieved through belt comparison. The belts in lane 1, 2, and 3 correspond to MB, DEMUX-IN 1, and DEMUX-IN 2 (Address input: A), respectively. The weak visibility of belts in lane 2 and 3 may be ascribed to the smearing of their G-rich Segments III in each of them. A new belt appeared in lane 4, which belongs to the MB DEMUX-IN 1 complex. MB and
DEMUX-IN 2 does not hybridize with each other, according to the smearing belt in lane 5, which can be ascribed to the overlap of MB and DEMUX-IN 2 belts according to their polarity.
The clear sharp belt in lane 6 belongs to DEMUX-IN 1-2 complex. Upon the presence of both inputs, two belts can be observed, corresponding to DEMUX-IN 1-2 complex and MB, respectively.
Figure S6. Native polyacrylamide gel analysis of the interaction among MB, EC-IN 0, EC-
IN 1, EC-IN 2, and EC-IN 3. EC-IN 0, EC-IN 1, EC-IN 2, and EC-IN 3 are abbreviated as EC
0, EC 1, EC 2, and EC 3, respectively. The sample in each lane and the identities of the main belts are indicated above and at the sides of the gel image, respectively.
Discussion of Figure S6 :
PAGE analysis of the interactions among the five DNA strands (MB and four inputs) in the
4-to-2 encoder is achieved through belt comparison. The belts in lane 1, 2, 3, 4, and 5 correspond to MB, EC-IN 0, EC-IN 1, EC-IN 2, and EC-IN 3, respectively. The weak visibility of belts in lane 2 and 4 is ascribed to short strand of EC-IN 0 and EC-IN 1. The weak visibility of belts in lane 4 and 5 may be ascribed to the smearing of their G-rich section in either of them. Only in lane 8 and 9, new belts appeared, which belong to MB EC-IN 1 complex and MB EC-IN 3 complex, respectively.
Figure S7. FAM fluorescence spectra of (a) MB and (b) MB-MBC complex.
Figure S8.
Hybridization between MB and MBC. The two-base mismatch can be clearly seen.
Figure S9.
Fluorescence spectra of G-4/NMM complex generated from (a) DC-IN 0 without
Segment III plus and (b) DC-IN 0.
Figure S10.
Native polyacrylamide gel analysis of the interaction among MB, MBC, DC-IN
0, and DC-IN 1. The sample in each lane and the identities of the main belts are indicated above and at the sides of the gel image, respectively.
Discussion of Figure S10 :
PAGE analysis of the interaction among the four DNA strands in the 2-to-4 decoder is achieved through belt comparison. The smearing in lane 3 can be ascribed to the existence of Grich section in DC-IN 0. There are two belts in lane 4, the up one of which belongs to the selfhybridization dimer of DC-IN 1 and the other comes from single DC-IN 1 strand. The new belt in lane 5 confirms the hybridization between MB and MBC. When DC-IN 0 appears in the MB-
MBC mixture (lane 6), the belt of MB-MBC disappears and MB belt can be found. The smearing in lane 5 is caused by the G-rich segments in MBC DC-IN 0 complex. DC-IN 1 is also able to disturb the interaction between MB and MBC. This is indicated in lane 7, where MB belt and new belt belonging to MBC DC-IN 1 complex can be observed. DC-IN 0 and DC-IN 1 can hybridize with each other to form a stable complex that is the new belt in lane 8. The complex is also the main product in lane 9 and 10, where there is also a small new belt, assigned to the fourcomponent complex consisting of two MBC, DC-IN 0 and DC-IN 1 as the belts of MBC disappears. In lane 10, the MB belt re-appears due to the liberation from MB-MBC complex.