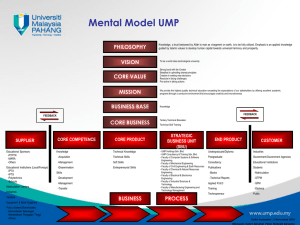
Final Summary Report, European Nuclear
advertisement

FINAL SUMMARY REPORT EUROPEAN NUCLEAR THERMODYNAMIC DATABASE (ENTHALPY Project) CO-ORDINATOR Dr. A. DE BREMAECKER Detached from SCK.CEN-Mol at IRSN/DRS/SEMAR CEN de Cadarache Bât 702 BP 2 F - 13108 Saint-Paul-lez-Durance FRANCE Tel.: + 33 4 4225 3501 Fax: + 33 4 4225 2929 LIST OF PARTNERS 1. IRSN/DRS, Cadarache, France 2. CEA/DRN-Grenoble, France 3. AEA-Technology,Harwell, United Kingdom 4. THERMODATA, Grenoble, France 5. FRAMATOME-ANP, Erlangen, Germany 6. CEA-DRN-DTP, Cadarache, France 7. EdF, Clamart, France 8. AEKI-KFKI, Budapest, Hungary 9. SKODA-UJP, Praha, Czech republic 10. SCK.CEN, Mol, Belgium 11. ULB, Université Libre de Bruxelles, Brussels, Belgium 12. UCL, Université Catholique de Louvain, Louvain-la-Neuve, Belgium CONTRACT N°: FI3S-CT1999-00001 EC Contribution: Partners Contribution: Starting Date: Euro 600 000 Euro 1.125.000 February 2000 Duration: 36 months FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 CONTENTS LIST OF ABBREVIATIONS AND SYMBOLS EXECUTIVE SUMMARY A. OBJECTIVES AND SCOPE B. WORK PROGRAMME C. B.1 Assembling of the two existing databases & extension to new elements B.2 Separate Effect tests B.3 Improvement and validation of the database B.4 Evaluation of the consequences of the uncertainties B.5 Methodologies of coupling the database with SA codes B.6 Edition of the database WORK PERFORMED AND RESULTS C.1 State of the Art Report C.2 Assembling and extension of the database (WP 1) C.2.1 Introduction C.2.1.1 C.2.1.2 C.2.1.3 C.2.1.4 Selection of the elements to be included in NTD Modelling and merging procedure Selection of the systems to be included in NTD Critical assessment work C.2.2 Critical assessment and assembling (Task 1.1) C.2.3 Extension of the database (Task 1.2) C.2.3.1 C.2.3.2 C.2.3.3 Extension of NTDIV01 to Boron and Carbon and assembling of the final In-Vessel Nuclear Thermodynamic database : NTDIV02 Extension of NTDIV02 to concrete elements (Al, Ca, Mg, Si). Assembling of the final In/ex Vessel Nuclear Thermodynamic Database : NUCLEA Comparison calculations FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 2 C.2.4 State of the validation C.3 Separate Effect Tests (WP 2) C.3.1 Solidus – Liquidus temperatures for (U,Zr)O2+x (Task 2.1) C.3.1.1 C.3.1.2 C.3.2 Tliq in Zr-Fe-O & Zr – Cr – O systems (Task 2.2) C.3.2.1 C.3.2.2 C.3.3 Experimental Results Experimental Results Tliq in B2O3 + FeOx/ZrO2/UO2 systems (Task 2.3) C.3.3.1 C.3.3.2 C.3.3.2.1 C.3.3.2.2 C.3.3.2.3 Experimental Results Fe2O3-B2O3 system ZrO2-B2O3 system UO2-B2O3 system C.3.4 Tliq in UO2-ZrO2-FeOx-CaO-Al2O3-SiO2 systems (Task 2.4) C.3.5 Experimental study of (sub)system(s) in UO2-ZrO2-BaO-MoOx (Task 2.5) C.3.5.1 C.3.5.1.1 C.3.5.1.2 C.3.5.1.3 C.3.5.1.5 Direct determination of the phase diagram (Task 2.5.2.1) Experimental Results in the ternary system BaO-ZrO2-MoO3 Results in the quaternary system UO2-BaO-ZrO2-MoO3 in reducing atmosphere at 1480°C and 1600°C Results in the quaternary system UO2-BaO-ZrO2-MoO3 in oxidising atmosphere at 1600°C Conclusion C.3.5.2 C.3.5.2.1 C.3.5.2.2 Release in thermal gradient (Task 2.5.2.3) Experimental results C.3.5.3 Review of the U-Zr-Mo-Ba-O system including in irradiated fuel pins (Task 2.5.2.4) Solid solubility and precipitation of FP Molybdenum Solid solubility and precipitation of FP Baryum and Zirconium Post-irradiation observations Review of equilibria established at the Mo/MoOé equilibrium Conclusions C.3.5.1.4 C.3.5.3.1 C.3.5.3.2 C.3.5.3.3 C.3.5.3.4 C.3.5.3.5 FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 3 C.4 Validation and Improvement of the database (WP 3) C.4.1 Validation based on fuel experiments and empirical models (Task 3.1) C.4.2 Validation based on experiments (task 3.2) C.4.3 Validation based on VULCANO/CEA and COMETA/NRI experiments (Task 3.3) C.4.3.1 C.4.3.2 C.4.3.3 C.4.4 Improvement by literature review (task 3.4) C.4.4.1 C.4.4.2 C.4.5 VULCANO VE-U3 experiment VULCANO VE-U7 COMETA / NRI test Boiling points and vapour pressures of the elements Thermodynamic properties of UxOy gaseous species Validation based on Tliq and Tsol measurements in UO2+x-ZrO2 (AEA-T) and Fe-Zr-O (SKODA), and global experiments (VERCORS, Hofmann) (Task 3.5) C.4.5.1 C.4.5.2 C.4.5.3 C.4.5.4 UO2+x-ZrO liquidus and solidus measurements Fe-Zr-O liquidus and solidus measurements Fission Products release (Vercors tests) Corium pool stratification involving Fe-O-U-Zr (+ Cr, Ni) C.5 Influence of uncertainties C.6 Coupling methodologies to SA codes C.6.1 Coupling methodology of thermodynamic databases to SA codes (CROCO and ICARE/CATHARE) (Task 5.1) C.6.1.1 C.6.1.2 C.6.1.3 C.6.1.4 C.6.1.5 C.6.2 Tabulation of the phase diagram Interface with the thermochemical code Generation of the library, research and interpolation in the library Applications Time consuming and coupling aspects Recalculation of TMI2 with MAAP4 and the database (Task 5.2) C.6.2.1 C.6.2.2 C.6.2.3 Thermo-chemistry of the U-Zr-O domain Recalculation of the U-Zr-O diagram using the NUCLEA database Recalculation of TMI-2 using the new Tliq and Tsol C.6.3 Simplification of the database and/or of the equilibrium code and adaptation to SA codes (Task 5.3) C.7 Edition of the NUCLEA database FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 4 CONCLUSION REFERENCES TABLES FIGURES LIST OF ABBREVIATIONS & SYMBOLS CIT Corium Interactions and Thermochemistry COLOSS Core Loss DTA Differential Thermal Analysis EDX Energy Dispersive Microanalysis EPMA Electron Probe Micro Analysis FP Fission Product ICP Inductively Coupled Plasma LOCA Loss of Coolant Accident MDB Material Data Bank ( module of the ASTEC System Code) MCCI Molten Corium-Concrete Interaction NTD Nuclear Thermodynamic Database SA Severe Accident SEM Scanning Electron Microscopy SGTE Scientific Group Thermodata Europe TDBCR-IV Thermodynamic Data Base Corium - In Vessel THMO Thermo-chemical Modeling and data TMI-2 Three Mile Island Unit 2 VPA Visual Polythermal Analysis XRD X-Ray Diffraction FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 5 EXECUTIVE SUMMARY ENTHALPY is a shared-cost action where 11 partners from 6 countries succeeded to pro- duce one unique well validated thermodynamic database for in- & ex-vessel applications. The first step was the critical assessment and merging of the two thermodynamic databases (THERMODATA/Grenoble and AEA-Technology/Harwell) in one database called "NTDiv" (for Nuclear Thermodynamic Database in-vessel). The second step was the extension of the database to Boron and Carbon, leading to the edition of the NTDiv0201 version (14 elements). In a third step, four ex-vessel elements and four new oxides components were added. All together, the final database is based on 20 elements : O-U-Zr-Ag-In-Fe-Cr-Ni-Ba-La-Ru-Sr-B-C-Al-Ca-Mg-Si + Ar-H and includes in particular the 15 oxides system : UO2-ZrO2-FeO-Fe2O3-Cr2O3-NiO-In2O3-BaO-La2O3-SrO-B2O3-Al2O3-CaO-MgO-SiO2 In view of the extension to Boron, AEKI determined experimentally 3 new phase diagrams namely ZrO2-FeOx, ZrO2-B2O3 and B2O3 – UO2. About the open question of the solubility of zirconia in Zr-Fe, SKODA tests on Tliq and Tsol in the corner of low oxygen in the Zr-Fe-O system confirmed the large solubility. The influence of the hyperstoichiometry of UO2 on Tliq and Tsol of typical corium (U,Zr)O2 was tested by AEA-T and the results confirmed the model used in the database. The direct construction of the complex phase diagram U-Zr-Ba-Mo-O was made in different atmospheres (reducing conditions, and in air) as an exploratory research and showed that in rather oxidizing atmospheres, the stability of the Ba zirconates decreases in favour of Ba molybdates or scheelite (BaMoO4). This was confirmed by tests in thermal gradients at UCL and by PIE at SCK.CEN on irradiated fuel pins at high burn-up. Tliq of 10 ex-vessel mixtures (sub-systems in UO2-ZrO2-FeOx-CaO-Al2O3-SiO2) measured by different techniques, confirmed or validated points of the ex-vessel database. The database was further improved by THERMODATA. The thermodynamic properties of 11 gaseous elements and of the UxOy species were specially revisited and reassessed. The database was validated by pre- and post-calculations of experimental tasks, and by (semi)global tests : FP release (VERCORS tests), in-vessel corium pool stratification (SASCHA), and ex-vessel corium spreading (VULCANO). The influence of uncertainties on the corium physical properties was studied on the point of view of the variability of key properties from different versions of the database. A strategy was developed by IRSN to couple the database to severe accident codes, through tabulation of the phase diagram, generation of a library by GEMINI2 and interpolation. This was proven to be efficient for the 4 elements system U-Zr-Fe-O. A reactor calculation (TMI2) on the new subsystem U-Zr-O did by EdF with MAAP4 concluded that the calculation must be enlarged to a subsystem including at least iron. The “NUCLEA” database, edited by Thermodata is now commercially available. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 6 A. OBJECTIVES and SCOPE For the prevention, mitigation and management of severe accidents, many problems related to core melt have to be solved : fuel degradation, melting and relocation, convection in the core melt(s), coolability of the core melt(s), fission product release, hydrogen production, ex-vessel spreading of the core melt(s), etc. To solve these problems such properties like thermal conductivity, heat capacity, density, viscosity, evaporation or sublimation of melts, the solidification behavior, the tendency to trap or to release the fission products, the stratification of melts, must be known. However most of these properties are delicate to measure directly at high temperature and/or in the radioactive environment produced by the fission products. Therefore some of them must be derived by calculations from the physical-chemical description of the melt : number of phases, phase compositions, proportions of solids and liquids and their respective oxidation state, miscibility of the liquids, solubility of one phase in another, etc.. This information are given by the phase diagrams of the materials in presence. The phase diagrams can be experimentally determined in specific tests, or they can be constructed by calculations made on the basis of measurements of the Gibbs energies, binary or ternary interaction parameters, and models (the « associated » model or the « ionic » model) and in a second step validated by application to global tests. Results of the “CIT” & “THMO” projects (4th R&D-FW Program) were previously obtained in this field but had to be confirmed and broadly enlarged. In this frame, the general objective of “ENTHALPY” was to obtain one unique European commonly agreed thermodynamic database for in- and ex-vessel applications, well validated and with methodologies able to couple the database to Severe Accident codes used by end-users like utilities, safety Authorities and nuclear designers. B. WORK PROGRAMME The work programme was organised in 6 Work Packages (WP) described below (see also Table I). Each WP contains one or more tasks. Each task was performed by one partner. At the mid-term assessment meeting it was agreed to merge two tasks (formerly named "Task 2.2.3 : Tliq and Tsol in UO2-ZrO2-FeO-Cr2O3" and "Task 2.4 : UO2-ZrO2/(CaO+Al2O3) both performed by FRAMATOME ANP) , in one task named "Task 2.4 : Tliq in UO2-ZrO2-FeOxCaO-Al2O3-SiO2" entirely performed by FRAMATOME ANP, and task 2.2.2 and 3.2 in order to re-in force the effort on the key Zr-Fe-O system (Task 2.2.1). B.1 : Assembling of the two existing databases and extension to new elements (WP1) The two existing nuclear thermodynamic databases had to be merged in one database, with agreed thermodynamic models, covering the entire field from metal to oxide (borides/carbides) for a complex multi-component chemical system). FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 7 B.2 : Separate Effect Tests (WP2) Tests were performed in the key U-Zr-Fe-O (sub)systems and in other in- & ex-vessel subsystems (B2O3, B, Pu, PuO2, Mo ; Si, SiO2, Ca, CaO, Al2O3 etc) including FPs with high decay heat (Ba), in such a way that thermodynamic results (Tliq, Tsol, enthalpies, solubility limits) were obtained. B.3 : Improvement and validation of the database (WP 3) This WP included the improvement and validation of the new database against global tests. B.4 : Evaluation of the consequences of the uncertainties (WP 4) This WP envisaged the consequences of uncertainties on Corium physical properties. B.5 : Methodologies of coupling the database with SA codes (WP 5) Methodologies were developed to effectively couple the database to severe accidents codes, and a recalculation of TMI2 with MAAP4 was made. B.6 : Edition of the database (WP 6). This WP included the documentation, the discussion of an agreement on property rights and the diffusion of the database. C. WORK PERFORMED AND RESULTS C.1 State of the Art Report Before the start of ENTHALPY, THERMODATA, had developed the specific TDBCR thermodynamic database for nuclear safety applications. It covered the entire field from metal to oxide domains in the following multi-component system : O-U-Zr-Fe-Cr-Ni-Ag-In-Ba-La-Ru-Sr-Al-Ca-Mg-Si + Ar-H and was able to be used for both in- or ex- Vessel applications. AEA-T, through external collaborations, had developed a large oxide thermodynamic database, more oriented to Ex- Vessel applications, based on the following oxides : UO2-ZrO2-Fe2O3-BaO-La2O3-CeO2-Ce2O3-SrO-Al2O3-CaO-MgO-SiO2. Specific databases were also available (metal domain : U-Fe-Zr-Si-Ba-Sr-Ce-La-Ru-Te where Cerium was introduced for simulating Plutonium ; metal oxide field : U-O-Zr-Si). These two databases needed to be improved, specially in some key-subsystems (U-O, Zr-FeO for instance), to be extended to the absorber elements Boron and Carbon, and to be more largely validated. The coupling of the database to SA codes was also weak. Faster and more efficient methodologies of coupling were needed. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 8 C.2 Assembling of the database (WP 1) C.2.1 Introduction The development of a common database, based on the CALPHAD method, involved the definition of a precise methodology to assemble the data coming from the two sources. An inventory of the content of the two databases was made and discussed. Important decisions were taken concerning : - the elements to be included in the database, - the subsystems (binary, quasi-binary, ternary, quasi-ternary) to be critically assessed, - the standards to be adopted (thermodynamic data for unary systems, thermodynamic models). C.2.1.1 Selection of the elements to be included in NTD The following elements (18 + 2) were selected for being included in NTD : O-U-Zr-Fe-Cr-Ni-Ag-In-Ba-La-Ru-Sr-B-C-Al-Ca-Mg-Si + Ar-H These are the elements of the main materials involved in a severe accident : UO2 (fuel), Zr (zircaloy cladding), Fe-Cr-Ni (steel of the structural components), SIC and B4C (control rods), Ba-La-Ru-Sr (selected fission products), Al2O3-CaO-MgO-SiO2 (concrete), H2O (water), O (air, oxides). Argon was added only as a neutral species in the gas phase. Hydrogen has not been taken into account in non stoichiometric solution phases. As any metallic element can be oxidised at a given oxygen potential, the multicomponent (15) oxide system is also a subset of the whole database : UO2-ZrO2-FeO-Fe2O3-Cr2O3-NiO-In2O3-BaO-La2O3-SrO-B2O3-Al2O3-CaO-MgO-SiO2. (AgO and RuO2 are two oxides decomposed before melting.) C.2.1.2 Modelling and merging procedure The development of a thermodynamic database for multi-component system is based on the critical assessment of the most relevant sub-systems (binary, ternary, …) i.e. the compilation of the available experimental data (phase diagram and thermodynamic properties), the inventory of all possible phases, the choice of suitable thermodynamic models for each phase, and finally, the optimisation of Gibbs energy parameters. The whole subset of Gibbs energy parameters for a given sub-system is also called a thermodynamic database, and allows the user to re-calculate the whole phase diagram. The list of all possible condensed solution phases was commonly built, and for each identified solution phase, a thermodynamic model was proposed and agreed. Common standards were also adopted for unary systems (pure elements and oxides). C.2.1.3 Selection of the systems to be included in NTD The thermodynamic modelling of the selected multi-component system was based on the critical assessment of all the possible binary (153) or pseudo-binary (105) systems. Only the most important ternary or pseudo-ternary systems (metal, metal-oxygen, oxide) were critically assessed, due to the very high number of possible ternary systems. C.2.1.4. Critical assessment work For each system, the following points were carefully treated : - The list of bibliographic references has been up-dated. - The set of experimental data was re-checked. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 9 - The new experimental information was included in the optimisation process - The heat capacity of stoichiometric compounds was estimated, in order to produce fundamental values for substances ( H°298.15 K, S° 298.15 K, Cp(T), G - HSER). - A new optimisation was performed in order to correct any evident disagreement between calculated and experimental values - The calculated and experimental temperatures and compositions of the invariant reactions or specific points have been compared in numeric tables. - The phase diagram and specific thermodynamic properties were calculated and compared to experimental values taken from literature on figures. - A new set of optimised Gibbs energy parameters has been produced and included in the new nuclear thermodynamic databases (NTDIV01, NTDIV02, NTD). - The lack of experimental knowledge was identified - Finally, a new quality criterion was proposed for each sub-system. C.2.2 Critical assessment and assembling (Task 1.1) (Assembling of a first partial In-Vessel Nuclear Thermodynamic Database : NTDIV01) A partial thermodynamic database for In-Vessel applications, named NTDIV01, was first assembled, based on 14 elements : O-U-Zr-Ag-In-Fe-Cr-Ni-Ba-La-Ru-Sr + Ar-H It included in particular the 10 oxides system : UO2-ZrO2-FeO-Fe2O3-Cr2O3-NiO-In2O3-BaO-La2O3-SrO. Then the Gibbs energy parameters of all possible phases were assembled by using the thermodynamic modelling of the Gibbs energy from the assessed binary and ternary subsystems in the GEMINI2 code format (Ref. [1] & [2]). NTDIV01 is composed of three different files : 1. The Gibbs energy parameters of the « lattice-stabilities », i.e. the Gibbs energy difference of each element of the multi-component system in a given structure and a reference one (SER = Standard Element Reference). 2. The Gibbs energy parameters of all possible substances referred to any chosen reference state, provided that it is present in the first file 3. The Gibbs energy parameters of solution phases. C.2.3 Extension of the Database (Task 1.2) C.2.3.1 Extension of NTDIV01 to Boron and Carbon and Assembling of the final InVessel Nuclear Thermodynamic Database : NTDIV02 NTDIV01 was extended firstly to two new elements B and C and one oxide B2O3 to the database. After assembling, the final thermodynamic database for In-Vessel applications, named NTDIV02,was thus based on 16 elements (O-U-Zr-Ag-In-Fe-Cr-Ni-Ba-La-Ru-Sr-B-C + Ar-H), and included in particular the following 11 oxides system (UO2-ZrO2-FeO-Fe2O3Cr2O3-NiO-In2O3-BaO-La2O3-SrO-B2O3). This extension involved the thermodynamic modelling of new systems. Some of them were already accepted : B-Fe, B-Ni, C-Cr, C-Fe, C-Ni, C-Zr, C-Cr-Fe, C-Cr-Ni, C-Fe-Ni, CrFe-Ni, C-Cr-Fe-Ni. Other ones, as B-Cr, B-O, B-U, C-U, B-Zr, B-C, B-C-Fe, B-C-U, B-C-Zr, B-Fe-U, B-Fe-Zr, C-O-U, C-O-Zr, C-U-Zr, C-O-U-Zr, were modelled by THERMODATA. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 10 35 new binary and 13 ternary systems were re-assessed, AEA-T being more involved in oxidic systems including B2O3, THERMODATA in metallic ones. The oxide systems including B2O3 ,-BaO, -Cr2O3 , -FeO, -Fe2O3, -In2O3, -La2O3, -NiO, SrO, -UO2, -ZrO2 and -FeO-Fe2O3, were made by AEA-T (Ref. [3]). Experimental data for the pseudo-binary systems B2O3 with -FeO, -Fe2O3, -UO2, -ZrO2 came from Task 2.3 (Figure 1). 12 other binaries were assessed : Al-B, Al-C, B-Ba, Ba-C, B-In, C-In, B-La, C-La, B-Ru, CRu, B-Sr, and C-Sr by THERMODATA (Ref. [4]). The two important binary systems B-U and C-U were optimised (Ref. [5]) and the model extended in the quaternary system C-O-UZr. NTDIV02 was assembled by the CALPHAD method on the basis of 91 binaries, 55 pseudo-binaries and 21 ternary systems. It contains 35 solution phases,191 reference substances, 203 substances and 148 gaseous species (Ref. [6]). C.2.3.2 Extension of NTDIV02 to concrete elements (Al, Ca, Mg, Si). Assembling of the final In/Ex Vessel Nuclear Thermodynamic Database : NUCLEA INTDIV02 was extended to the concrete elements for ex-vessel applications, adding four new elements Al, Ca, Mg and Si, and four new oxide components, Al2O3, CaO, MgO and SiO2. The final database named NTD and later “NUCLEA”, is based on 20 elements : O-U-Zr-Ag-In-Fe-Cr-Ni-Ba-La-Ru-Sr-B-C-Al-Ca-Mg-Si + Ar-H and includes in particular the 15 oxides system (Ref. [7]) : UO2-ZrO2-FeO-Fe2O3-Cr2O3-NiO-In2O3-BaO-La2O3-SrO-B2O3-Al2O3-CaO-MgO-SiO2 23 binary or pseudo-binary systems were identified - either to be checked : Al-Cr, B-Si, Ba-Si, Ca-La, Ca-Si, La-Si, Mg-O, Al2O3-BaO, Al2O3NiO, CaO-”FeO”, Fe2O3-O2Si, MgO-O2U, OSr-O2Si - or to be re-assessed : O-Si, Si-Sr, Fe2O3-MgO, CaO-UO2 - or to be done : B-Ca, C-Ca, B-Mg, C-Mg, Al2O3-Fe2O3, B2O3-MgO. 9 ternary systems were included, either already available as : Al2O3-CaO-O2Si, Al2O3-O2Si-O2U, Al2O3-O2Si-O2Zr, Al2O3-O2U-O2Zr, O2Si-O2U-O2Zr or recently made as : Al2O3-B2O3-CaO, Al2O3-B2O3-O2Si, B2O3-CaO-O2Si, B2O3-FeOx. 9 ternary oxide systems were directly calculated from the binaries, but ternary interaction parameters should be assessed in the future : Al2O3-CaO-FeO, Al2O3-CaO-Fe2O3, Al2O3-Fe2O3-SiO2, CaO-FeO-Fe2O3,CaO-FeO-O2Si, CaO-Fe2O3-O2Si, FeO-Fe2O3-O2Si. The following systems were evaluated or re-evaluated : Al2O3-B2O3, B2O3-CaO, B2O3O2Si, Al2O3-B2O3-MgO, B2O3-CaO-MgO, B2O3-MgO-O2S. The experimental state of the art and thermodynamic modelling of the O-U binary system (Figure 2) was improved using a sub-lattice model to describe the UO2+x phase (Ref. [8]). C.2.3.3 Comparison calculations Calculations for selected oxide systems were performed and the results compared with those obtained using a commercial database. The latter called MTOX has been produced as FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 11 part of a collaborative project by the NPL (National Physical Laboratory, UK) and other industrial sponsors. The current database includes oxide systems for a range of components (e.g.K, Na, Fe, Ca, Si, Al, Mg) and has been well validated. Calculations of oxide systems using the MTOX database and the Gibbs energy minimisation code MTDATA were carried out (Ref. [9]). The system FeO-Fe2O3-CaO-SiO2 was selected for the comparison exercise and 21 ternary and quaternary compositions proposed by IRSN. The MTOX results showed that in some cases the change in the amount of liquid phase is gradual over the temperature range whereas in others the amount increases very rapidly over a temperature rise of a few degrees. These results are to be compared with calculations performed by IRSN using NUCLEA and the GEMINI code. Previous code comparison studies have shown that the results produced by the different equilibrium codes (MTDATA and GEMINI) using the same database are in good agreement. C.2.4 State of the validation A quality criterion was established for each assessed sub-system: * Estimated ; No experimental data available. ** Perfectible ; Some domains need more experimental information (phase diagram or thermodynamic properties). *** Acceptable ; The system is well known and satisfactorily modelled. **** High quality ; The system is quite known and modelled. The complete list of binary systems based on pure elements and oxide pseudo-binary systems based on pure oxides are presented in (Tables II and III) respectively. Due to the very high number of possible ternary (816) and pseudo-ternary (455) systems only the most important ternary systems for practical applications (Tables IV and V) were assessed at this time. C.3 Separate Effect Tests (WP 2) C.3.1 Solidus – Liquidus temperatures for (U,Zr)O2+x (Task 2.1) In a severe accident the fuel in the corium will oxidise in steam, in particular for the exvessel sequence. No thermodynamic data exists for the pseudo binary system (U, Zr)O2+x . C.3.1.1 Experimental Tsol and Tliq measurements were measured by the thermal arrest. The PTE included ceramography and SEM analysis. The experimental set-up and proposed compositions were assessed first by the CEA based on preliminary calculations performed using the TDBCR-IV 992 database. Based on this assessment the compositions of the three samples finally investigated were UO2.00-ZrO2, UO2.08-ZrO2 and UO2.15-ZrO2 with the ratio of UO2/ZrO2 set to 80/20 wt.%. C.3.1.2 Results Tsol & Tliq of the three samples UO2.00-ZrO2, UO2.08-ZrO2 and UO2.15-ZrO2 were measured (Table VI) (Ref. [10]). The results indicate that the solidus-liquidus gap for the stoichiometric and hyperstoichiometric compositions is slightly larger than predicted and some modifications of the model for the U-Zr-O system are required. These modifications to the NUCLEA database have now been carried out. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 12 C.3.2 Tliq in Zr-Fe-O & Zr-Cr-O systems (Task 2.2) The objective is the experimental determination of Tsol (and Tliq) of one selected alloy in the Zr-rich corner of each of the Zr-Fe-O and Zr-Cr-O systems (Zr-Fe-O alloy near 82.317.3-0.4 wt% and one Zr-Cr-O alloy near 79.6-20.0-0.4 wt%). C.3.2.1 Experimental 6 Zr-Fe-O and 5 Zr-Cr-O alloys were prepared from pure metals (+ ZrO2). Their chemical composition was analysed, and their hardness (HV10) measured. The resistance furnace of the Balzers Exhalograph was adapted to the video-recording. This resulted in a successful off-line magnified viewing of the melting process on a computer screen. The camera & pyrometer were coupled with a PC-based image analyser. Tsol & Tliq could be approximately determined from the off-line sequential set of images. C.3.2.2 Results Tsol and Tliq were measured by both the first appearances of the liquid phase (solidus) and the complete melting manifested by formation of a drop (liquidus) (Figure 3 a, b). This method seems to locate the solidus and liquidus with a precision of ~ 15-25 C. Tsol agreed relatively well with the values calculated using the NTDiv01 database. The measured liquidus temperatures TLIQ for both systems were relatively close to Tsol. A question arose : does a solid phase exist in the interior of the presumed “molten” drop or not? To answer this question the Zr-Fe-O specimens were heated to various temperatures beyond TLIQ, i.e. between TLIQ and ~1700 C and cooled down as rapidly as possible (~11 C/s). The microstructure of the formed drops was investigated by both the optical metallography, by X-ray phase analysis and by microprobe analysis. The two main phases had an appearance of a liquid phase, the third one being cracks. A small black phase (less than 1% at TLIQ-CALC) is ~(Zr,Y)O2 originating from the Y2O3 support pad. In both specimens no ZrO2 was found. The oxygen was found only in solid solution, either within -Zr(O) containing small amount of iron or within the Zr-Fe matrix. Analysis of both the cooling curves (from ~1700 C) and the recorded images of the melting process also indicated that the main phases were liquid at TLIQ. SKODA concluded (Ref. [11]) that the specimens were completely molten at the liquidus temperature TLIQ (formation of spheres). This is probably valid for both systems Zr-Fe-O and Zr-Cr-O. C.3.3 Tliq in B2O3 + FeOx/ZrO2/ UO2 systems (Task 2.3) Boron trioxide has relatively low melting point (~450 oC), density and viscosity. Fused B2O3 readily dissolves many metal oxides may influence fuel degradation, melting and relocation processes. To model this influence, phase diagrams of B2O3 with Fe2O3, ZrO2 and UO2 are needed but were lacking. C.3.3.1 Experimental High purity powdered Fe2O3, ZrO2, UO2 and amorphous granulated B2O3 were used as starting materials. Due to the hygroscopic nature, boron trioxide was grinded to powder and dehydrated. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 13 Temperature history measurements with the test mixtures were carried out in a resistant furnace under a protecting gas. The liquidus temperatures are signalled by a characteristic brake on the temperature difference (T) curves of the cooling cycles. C.3.3.2 Results C.3.3.2.1 Fe2O3-B2O3 system The mean values of Tliq. are summarised in Table VII. Reproducibility of the data was estimated as ±10 oC. The phase diagram for the Fe2O3-B2O3 system could then be constructed (Figure 4), the phase relations bellow liquidus temperatures being taken from Makram (Ref. [12]). C.3.3.2.2 ZrO2 – B2O3 system The liquidus temperatures for the ZrO2-B2O3 system were determined by temperature history and as well as from solubility measurements (Ref. [13]). In the former experiments, test mixtures, containing 2.5 and 5.0 wt % ZrO2 were investigated. The results are given in Table VII. In the solubility experiments, the test mixtures containing ZrO2 in excess as compared with the saturated solution at a given temperature, were fused in a furnace at constant temperature. The original composition of the test mixture for both temperatures was chosen as 20 wt% ZrO2 – 80wt% B2O3. Because the density of ZrO2 (~5,6 g/cm3) is significantly higher than that of B2O3 (~1,5 g/cm ) , during the isothermal heating at the selected liquidus temperature there is stratification between the dense solid phase (a layer of unsolved ZrO2 deposited on the bottom) and the liquid phase (a saturated solution of ZrO2 in B2O3). Quenching allows to preserve the composition of the saturated liquid solution. This composition and the isothermal heating temperature (1800° and 2000°C) determine the position of the liquidus temperature on the phase diagram. They are given in Table VII. 3 The ZrO2 – B2O3 phase diagram (in the rich B2O3 side) is shown in Figure 5. C.3.3.2.3 UO2 – B2O3 system The liquidus temperatures for the UO2-B2O3 system were determined by temperature history measurements (Ref. [14]) and are summarised in Table VII. Combining these results with previous experimental results, the phase diagram could be constructed (Figure 5). C.3.4 Tliq in UO2 – ZrO2 – FeOx – CaO – Al2O3 – SiO2 systems (Task 2.4) The objective was to measure Tliq and possibly Tsol in systems relevant to SA ex-vessel sequences. 10 temperatures (Tliq and/or Tsol) were investigated by : Visual Polythermal Alalysis in an induction-melting furnace with a cold crucible (VPA IMCC), by VPA in the Galakhov micro-furnace, and by classical DTA (for solidus temperature determination). The compositions were discussed and defined together with the modelers (Table VIII). The knowledge of Tliq allows to compare the dissolution ability of different concretes to dissolve corium or to evaluate the viscosities of different concretes (Ref. [15]). FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 14 C.3.5 Experimental study of (sub)system(s) in UO2-ZrO2-BaO-MoOx (Task 2.5) NB : Task 2.5.1 was merged with Task 2.4. C.3.5.1 Direct determination of the UO2–MoO2–BaO-ZrO2 phase diagram (Task 2.5.2) The study is subdivided into 3 subtasks, performed resp. by ULB, SCK.CEN, UCL. C.3.5.1.1 Direct determination of the phase diagram ( Task 2.5.2.1) Two main steps were proposed in order to understand the mechanisms of the fuel degradation and the formation of the new phases as a function of oxygen potential : A. The study of the pseudo-ternary system BaO-ZrO2-MoOx ( with x = 0 ,2 ,3 ) in oxidising and reducing atmosphere giving a preliminary knowledge of the behaviour of the Mo0, Mo4+ and Mo6+ compounds in presence of BaO and ZrO2. B. The extension of the ternary system to the quaternary system UO2-BaO-ZrO2-MoOx at 1600°C at 3 different oxygen pressures, i.e.10-16 atm, 10-9 atm and 10-5 atm.. C.3.5.1.1.1 Experimental A major part of anneal experiments were performed in electrically heated furnaces. Preliminary works were performed in oxidising atmosphere (air and nitrogen. Tests under controlled atmospheres were performed in three tubular furnaces equipped either with a zirconia oxygen sensor, either with an H2O sensor and a classic air reference zirconia gauge operating at 700°C). Quenching was possible in two of these furnaces. Phase identification and composition analysis were performed by optical microscope, X-RD, X-Ray fluorescence, SEM with EDX and EPMA. Calculations based on H2/H2O equilibria in order to check whether the effective pO2 over samples heated at 1400°C-1600°C correspond to those measured in the outlet part of the furnace (700°C) with the zirconia sensor, demonstrated that H2/H2O equilibria are immediately reached in all parts of the furnace so that the measured pO2 have to be corrected at 1400°C-1600°C, owing to higher water dissociation at those temperatures. Thus, the pO2 values measured at 700°C(10–24 atm, 10–17 atm and 10–13 atm )* correspond actually to 10–16 atm, 10–9 atm and 10–5 atm at 1600°C. C.3.5.1.1.2 Results in the ternary system BaO - ZrO2 – MoO3 a) In oxidising conditions (pO2> 10-4 atm, T<= 1600°C), BaO reacts preferably with MoO3 to yield BaMoO4 (scheelite). For Ba/Mo ratios >1, BaO reacts with the phases BaZrO3 and BaMoO4 to form other Ba-rich zirconates and molybdates of higher Ba order like Ba3Zr2O7, Ba2ZrO4, Ba2MoO5 and Ba3MoO6. For the identified Mo-compounds, the stable Mo valence is 6+; therefore solid solutions between Zr4+ and Mo6+ are limited : no Mo detected in BaZrO3; and a maximum of 5,5 mol% of Zr in substitution for Mo in sheelite BaMoO4. b) In reducing conditions (10-20 <= pO2 <=10-17 atm, 1200°C <= T <= 1500°C), in Mo-rich compositions, the Mo compounds are reduced into Mo0 so that BaO reacts preferably with ZrO2. The perovskites compounds BaZrO3 and BaMoO3 form solid solutions BaMo1-yZryO3 with y = 0,1 and BaZr1-xMoOxO3 with x = 0,08 at 1200°C. The thermal evolution shows that the major part of BaMoO3ss disappears according to a disproportionate reaction yielding Mo0 in a dispersed phase and Mo6+ in Ba3MoO6 while the remainder Mo+4 is stabilised only in BaZrO3 ss. As a function of composition and temperature, Mo0, Mo4+ and Mo6+ compounds can thus co-exist in strongly reducing conditions. BaO volatilisation in Ba-rich compositions C and D was not observed at 1400°C. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 15 Results in the quaternary system UO2 - BaO - ZrO2 – MoO3 in reducing atmosphere : 1480°C/ 10-17 atm2 pO /8 & 32 h ; and 1600°C/ 10-16 atm pO2/8h The main stability domains evidenced from the identified compounds UO2ss, ZrO2ss, 0 Mo and the perovskite phase BaZrO3ss or BaUO3ss are depicted in Figure 6. BaO reacts preferably with ZrO2 to form the perovskites BaZrO3ss when BaO/ZrO2 2 and BaUO3ss when BaO/ZrO2 2. Mo : appears as a dispersed Mo0 metallic phase in equilibrium with Mo4+ present mainly in the BaZrO3ss (max 6.8 % in composition A) and BaUO3ss perovskite phases. Mo0 can dissolve up to 1 at%Ba, 1.5 at%Zr and 0.5 at%U. Its oxygen content (up to 14%) is probably related to oxidation upon cooling. There is no stable phase of molybdate. UO2 matrix forms a solid solution UO2-ZrO2 which may contain up to 14 % ZrO2 at 1480°C and 26.6% ZrO2 at 1600°C. When the concentration of Ba increases, the solubility of ZrO2 in UO2 gets reduced because of the formation of BaZrO3 ss. The UO2 matrix dissolves a very low quantity of Ba (<1 at%) and of Mo (<2 at%). ZrO2 : exists as a solid solution ZrO2-UO2 up to 12.8 mol% UO2 in sample F at 1600°C. Perovskite phases BaZrO3 “or BaUO3” : The coexistence of BaZrO3ss and BaUO3ss established at 1480°C is no longer observed at 1600°C where there is a complete miscibility of barium uranates and zirconates in each other. At 1600°C in sample A (with Ba/Zr = 1), the only BaZrO3 phase dissolving up to 5,8 mol% U and 6.8 mol% Mo is observed. For much higher Ba content (samples C and D), perovskites of uranates are found. Therefore, as in the samples as well as in irradiated nuclear fuel BaO is never exceeding the ZrO2 nor UO2 content, the volatility of BaO in reducing conditions is limited by its interactions with UO2 and ZrO2 (contrarily with the ternary observations). C.3.5.1.1.3 Thus, the major effect of the UO2 matrix is to reduce the stability of the Ba molybdates : these are reduced in Mo0 and Mo4+ with an enrichment of the Ba zirconates in U and formation of Ba uranates. C..3.5.1.1.4 At 1600°C in oxidizing atmosphere (pO2 10–9atm) Stability domains evidenced from the identified compounds UO2ss, ZrO2ss, BaMoO4 (scheelite), BaZrO3ss, the MoO2-rich phase and a new uranate (Ba2U3Ox by SEM) are drawn in Figure 6B. BaO reacts preferably with oxidised Mo to give BaMoO4 (already observed in the ternary system at pO2 >10–4 atm) which is present in all the nine compositions studied. Owing to its congruent melting at 1457°C UO2 dissolves a greater amount of ZrO2 (32% in sample F) than in strongly reducing medium. The content of Mo and Ba in this phase is low (1% and 0.3% resp.) ZrO2 in excess of its solubility limit in UO2 exists as a free phase of zirconia (dissolving 12.3% of UO2) in equilibrium with the matrix. Mo : All Mo compounds are oxidised at this oxygen pressure. The perovskite phase is limited to a Zr-rich solid solution occuring for Ba/Mo ratios >1, in a domain including compositions B and G. In this domain, BaZrO3ss contains 18.4 at% BaUO3 and 1.7 at% BaMoO3. The uranate phase appears in a large domain where Ba/Mo >1 (compositions B, G, C and D). This relatively homogeneous uranate whose composition is close to Ba2(U3-xZrx)O9+y is unknown in the more recent JCPDS files but has been assigned to a fluorite – type structure . FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 16 C.3.5.1.5 Conclusion The major effect of increasing the pO2 from reducing (10-16 atm) to oxidizing conditions (10-9atm) is a reduction of the stability of the Ba perovskites in favour of the formation of stable phases scheelite (BaMoO4) and MoO2 when Ba/Mo < 1 or scheelite (BaMoO4) and a new uranate Ba2(U3-xZrx)O9+y when Ba/Mo > 1 (Ref. [16]). C.3.5.2 Release in thermal gradient (Task 2.5.2.3) C.3.5.2.1 Experimental The investigations covered the study of oxides of U, Zr, Ba and in strong temperatures gradients and in different atmospheres.Three different powders mixtures (A, D, F) were prepared : (mol%) : A : 80% UO2 + 7% Ba + 7% Zr + 7% Mo D: 80% UO2 + 18% Ba + 1% Zr + 1% Mo F: 80% UO2 + 1% Ba + 18% Zr + 1% Mo Two types of tests were performed : A) release of condensation particles by homogeneous heating, and B) differential heating (in strong temperature gradients) A) The pellets were heated up to 2400°C. Condensation particles were sampled by an impactor .Compositions and test conditions are given in Table IX. B) Tests in a strong temperature gradient were also performed in order to reveal possible migrations or segregation of the different elements inside the pellet matrices. Pellets A, D and F were submitted to a strong temperature gradient of approximately 1000-2000°C from side to side XRD, EDX analyses,SEM and neutron activation were performed on pellets before and after heating as well as on the condensed particles . C.3.5.2.2 Results A) Release from heated pellets Ba and Mo prevail in particles condensed from pellet A (rich in Ba and Mo). In the case of pellet D, Ba, is most abundant in condensed particles. No Zr has been found even in the case of pellet F, where Zr is abundant. In the released and condensed particles, Ba and Mo(O) are closely associated suggesting molybdates and as in the pellets, U segregates from Ba/MO, but no Zr was observed at all. B) Behaviour in temperature gradients (7,5 mm pellets) Grains grow up with temperature as a consequence of fusion-recristalisation. Starting from an homogeneous distribution of U, Zr, Ba and Mo before heating, Ba and Mo migrate to the hot zone and conversely, U migrates to (or concentrates in) the colder zone (Figure 8). There is a marked segregation between U and Ba-Mo. Mo and Ba are strongly associated suggesting the presence of molybdates (observed by XRD as BaMoO4, BaMo4O13, Ba3Mo18O28). Migration of Zr from the cold to the hot zones in pellet F is compensated by U. In hot zones of pellet F (2000°C), in opposition to the cold ones, U and Zr seem to be in close association, probably as a solid solution or as urinates (Ref. [17]). FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 17 C.3.5.3 Review of the U-Zr-Mo-Ba-O system including in irradiated fuel pins(Task 2.5.2.4) The objective is to study the phase formation and the stability of the phases formed by the FPs Ba, Zr and Mo in nuclear fuel, focused on the influence of the oxygen potential. C.3.5.3.1 Solid solubility and precipitation of FP Molybdenum In the actual fabrication of LWR fuels, UO2 is slightly hyperstoichiometric, and MOX is slightly hypostoichiometric. But during and after irradiation, the initial O/M ratio increases. Different methods to measure the real oxygen potential (or O/M ratio) were developed, notably through the analysis of the repartition of Mo as metallic precipitates and in oxides. Since the free energy of formation of MoO2 is slightly above that of nuclear fuel, the measurements of the activities of Mo and MoO2 could be a method to estimate the local oxygen potential of irradiated fuel although the basic assumption of the elevated solubility of MoO2 in the UO2 matrix was never confirmed. Instead subsequent measurements and theoretical work confirmed that Mo solubility limit is very low. The quantity of Mo dissolved in irradiated UO2 is overestimated because of the contribution of submicron metallic or oxide precipitates that are not resolved in SEM or TEM microscopes. Nevertheless the mechanism remains partly valid : when pO raises up, Mo disappears from metallic inclusions and is incorporated in the grey phases which are perovskite ABO3 precipitates with Ba and Zr for the A and B sites respectively. Therefore the equilibrium Mo + O2 MoO2 is established between the Mo activity in metallic precipitates to the Mo activity in the ABO3 grey phase. But these ABO3 particles remain too small to be analyzed directly. C.3.5.3.2 Solid solubility and precipitation of FP Ba and Zr The solid solubility of Ba in the oxide matrix of irradiated FBR fuel and of simulation specimens is lower than 1,6 wt%. In LWR fuels, the ceramic precipitates are too small to be quantitatively measured. The solubility limit of 0.2wt% for barium in oxide fuel is only reached at a BU in excess of 45GWd/tHM only recently reached in LWR (see Table X). At temperatures below 1200°C, the solubility of ZrO2 in UO2 is limited (<0.2wt%), but it increases above this temperature. At 1600°C, a maximum solubility of 27.5 mol% is reported. A continuous solid solution of ZrO2 – UO2 is obtained at temperatures >2285°C. C.3.5.3.3 Post-irradiation observations Given a Mo production rate of about 100ppm per GWd/tHM, Mo tends at any relevant BU (Table 10), to precipitate from the UO2 matrix. The pO in normal LWR conditions being lower than the Mo/MoO2 equilibrium, Mo is normally incorporated in metallic precipitates. For the FPs Ba and Zr, the solid solubility in UO2 of both elements is limited to about 2000ppm under normal LWR operating temperatures. The solubility limit of both elements will be reached at intermediate burn-up level (Table X). Moreover PIE of fast reactor fuels have shown that precipitation of Ba is enhanced in the presence of dissolved Zr and that the complex perovskite phase (Ba,Sr,Cs)(Zr,Mo,U)O3 is formed. As long as the cladding did not fail, the conditions inside the fuel pin remain sufficiently reducing to maintain Mo in the metallic precipitates; the ceramic precipitates are of the general compositions (Ba,Sr,Cs)(Zr,U)O3. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 18 C.3.5.3.4 Review of equilibria established at the Mo/MoO2 equilibrium The experiments on the quasi-ternary system UO2-ZrO2-BaO showed complete mixing of BaUO3 and BaZrO3 in any proportion. In the UO2-ZrO2 rich side a broad two phase field exists, with either UO2 in equilibrium with Ba(U1-xZrx)O3 or U1-xZrxO2 in equilibrium with BaZrO3. Since the production rate of the fission products Ba and Zr is roughly 2:1, it is most likely that BaZrO3 is formed and is in equilibrium with (U1-xZrx)O2. In fuels with low oxygen potentials, molybdenum will not be part of the ceramic precipitates. At 1700°C and at the oxygen potential of the Mo/MoO2 equilibrium , in presence of Mo, Ba(U1-xZrx)O3 solid solution can incorporate limited amounts of Mo and forms a Ba(U,Zr,Mo)O3 perovskite phase, and the solubility of BaMoO3 in Ba(U1-xZrx)O3 depends on the U:Zr ratio (see ( Task 2.5.2.1) Therefore at moderate oxygen potentials, when only part of the produced (Table X) Mo oxidizes, Mo is incorporated in the perovskite precipitates. When more Mo is oxidized, several ceramic phases will co-exist and the formation of Scheelite type BaMoO4 becomes possible, even when the global oxygen potential is still buffered by the Mo/MoO2 couple. Experiments of Pascoal showed that the sole increase of pO induces a major change in the ceramic precipitate stability : while at lower oxygen potentials Mo is in equilibrium with BaZrO3, at more elevated oxygen potentials, it is ZrO2 that is stable against BaMoO4. C.3.5.3.5 Conclusions (Ref. [18]) The oxygen potential controls the phase formation of the ceramic precipitates. At low to intermediate BU and at low pO, Ba remains dissolved in the fuel matrix. At more elevated BU and at low pO, because the fission yield of Zr is larger than that of Ba, the perovskyte phase BaZrO3 is the preferred phase for precipitation of the fission products in a UO2 matrix and molybdenum precipitates as a metal in metallic precipitates Mo-Pd-Rh-TcRu. The perovskyte BaZrO3 can dissolve important amounts of UO2 and various FPs. When the pO rises, molybdenum oxidizes into MoO2 which is primarily incorporated in ceramic precipitates BaZrO3 ; at a pO above that of the couple Mo/MoO2 scheelite BaMoO4 is formed. This was observed in a LWR MOX irradiated fuel pin (at BU = 56GWj/tM) The solubility limit of MoO2 in BaZrO3 also is modified : from 25mol% substitution of ZrO2 at low oxygen potentials, down to below the detection limit in more oxidizing conditions. C.4 Validation and Improvement (WP 3) C.4.1 Validation based on fuel experiments and empirical models (Task 3.1) The objective of the task is to compare the predictions of fuel hyperstoichiometry using the model in NUCLEA with the experimental data and other empirical models and thermodynamic databases adopted within the nuclear industry. The validation of the model adopted for UO2+x in the database was carried out over the temperature range 973 to 1973 K. Results Agreement of the calculated values of oxygen potential for UO2+x obtained using NUCLEA, in conjunction with GEMINI2, and the experimental data is very good across the temperature FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 19 range (Ref. [19]). For the single phase fluorite region, the calculations confirm the use of some of the empirical models of Lindemer and Besmann, and de Franco and Gatesoupe and show reasonable agreement between the different methods. The agreement with the Green and Leibowitz data (only intended for use with stoichiometric and hypostoichiometric compositions (UO2-x) at high temperatures) is logically poor for O/U ratios greater than ~2.05 and temperatures less than 1373 K. C.4.2 Validation based on experiments (Task 3.2) This task was dropped by a decision at the mid term assessment meeting (Ref. [20]). C.4.3 Validation based on Vulcano/CEA & COMETA/NRI experiments (Task 3.3) The VULCANO facility allows to heat up to 3000K and to melt roughly 100 kg of corium composed of representative materials : UO2, ZrO2, FexOy, etc. in various proportions, and to pour it on representative materials of ex-vessel core catchers or spreading areas. C.4.3.1 VULCANO VE-U3 experiment The VULCANO VE-U3 (final melt composition in wt% : 60 UO2 – 24,1 ZrO2 – 6 Fe3O4 – 0,8 Fe2O3– 8,2 SiO2- 0,7 CaO – 0,2 Al2O3) experiment has been analysed and compared with successive versions of the nuclear thermodynamic databases (Ref. [21]). One of the major qualitative differences between the experiments and the computations regards the computation of the so called chernobylite phase i.e. the zircon-coffinite, which is a silicate of mixed UO2 and ZrO2 : (ZrxU1-x)SiO4. These compounds were calculated by different versions of the THERMODATA database coupled with the GEMINI 2 code but not found by material analysis in the VE-U3 corium. For different quaternary systems of the exvessel corium, the uncertainties are important. For instance, in the quaternary system U-Fe-SiO, no dissolution of iron in urania-zirconia phases is considered in the databases whereas in fact iron was observed in the urania-zirconia phase. Similarly, in the (U-Zr-Si-O) and (U-FeSi-O) systems, there are uncertainties on the miscibility limits, particularly for the "chernobylite" phase (ZrxU1-x)SiO4 where the solubility limit, x, is not well known. Some authors report a value up to 5% molar. Up to version 981 included, dissolution of “USiO4” in zircon (ZrSiO4) was not considered in the TDBCR database. When the post-test analyses of VE-U1 showed evidences of the dissolution of uranium in zircon, i.e. the formation of chernobilyte, this dissolution was introduced in the version 992 of TDBCR with a large solubility limit : up to 30 %mol of uranium could be dissolved in zircon (Zr0.7U0.3 SiO4 compound. Such a high solubility seems incompatible with the observed absence of chernobylite in the VE-U3 samples. Now, for the reactor case a precise knowledge of the remaining free silica content is needed (Figure 9). Testing the TDBCR versions (98,99,00), differences can go up to 26% for the solid fraction at a given temperature. The last version is probably the most exact. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 20 C.4.3.2 VULCANO VE-U7 experiment VULCANO VE-U7 consisted in pouring corium over two parallel paths : one in zirconia, the second in concrete. After spreading the global composition of the melts are very similar : 54 UO2 – 32 ZrO2 – 7 FeO – 3 SiO2 – 2 CaO – 0,3 MgO – 0,4 Al2O3 (in wt%). FeO is partly reduced in Fe. This presence of some iron balls gives a valuable information on pO2. Thermodynamic calculations with the GEMINI2 code and the TDBCR001 database predicted correctly the major phases of corium, but some discrepancies were found with the minor phases (Fe-concrete mixtures) and the evolution of the mortar (Ref. [22]). C.4.3.3 COMETA / NRI test In ECOSTAR, mixtures of simulant corium of ZrO2 - Fe2O3 – concrete and prototypic corium systems (UO2-ZrO2-Fe2O3 –concrete) were melted above 2000 K in air. The main important results are (Ref. [23]): For the ZrO2-Fe2O3 pseudo-binary system, the existence of the monotectic point M implies a difference of 700 K for the “liquidus” temperature. In the ZrO2-FeOx-SiO2 system, a miscibility gap is observed between 2423 K and 2073 K whereas the thermodynamic data calculates a full liquid domain (Figure 10). In the UO2-ZrO2-Fe2O3 –concrete systems, a monotectic temperature was experimentally identified at 2223K whereas the calculated Tliq is 2200K. Between 2623K and 2223K, two liquids were observed whereas GEMINI 2 computed only one liquid phase. C.4.4 Improvement by literature review (Task 3.4) C.4.4.1 Boiling points and vapour pressures of the element Thermodynamic properties of all gaseous metallic elements were revisited and reassessed, mainly at high temperature. A survey of the boiling points of 11 metallic elements (Table XI) with the related vapour pressures was made (Ref. [24]). A critical assessment was used to optimise of the thermo-chemical properties of gases in consistency with SGTE data for condensed species. C.4.4.2 Thermodynamic properties of UxOy gaseous species The gaseous binary O-U system is complex. Tests to determine pvap over UO2 are very difficult because the non-congruent vaporisation of the ceramic and its non-stoichiometry. The specific heats were taken from the last work made in Glushko THERMOCENTER. After the analysis of more than 150 scientific papers concerning the composition of the gaseous phase in equilibrium with urania, a critical assessment and optimisation of the thermodynamic properties led to a new set of thermodynamic data. The validation of that new data was made by comparison between calculations and experiments (Figures 11, 12) . A new set of thermodynamic data are available for the OxUy gaseous species (Ref. [25]) but experimental information are still needed. C.4.5 Validation based on Tliq & Tsol measurements in UO2+x–ZrO2 (AEA-T) and Fe-Zr-O (SKODA), & on global experiments (VERCORS, Hofmann) (Task 3.5) (Ref. [26]) C.4.5.1 UO2+x – ZrO2 liquidus and solidus measurements FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 21 Pre-calculations were performed with the experimental conditions of the UO2+x – ZrO2 tests foreseen at AEA-T in closed cell, i.e. at constant volume, with x = 0 – to 0,10 (Task 2.1). All calculations were performed with the GEMINII2 code and 2 databases TDBCR-iv 992 and NTDiv01, able to take into account the UO2 hyper-stoichiometry. The pre-calculations made for the two extreme compositions proposed by AEA-T( x = 0 and 0,1) showed that an intermediate composition like x = 0.05 is not hyper-stoichiometric enough to be sensitive to a change of Tliq from the stoichiometric composition. Therefore the hyperstoichiometries tested by AEA-T were fixed to x= 0 - 0.08 - 0.15. The AEA-T test results are in general good agreement with the calculations (Figure 13) : the difference in liquidus temperature are less than 50K which is acceptable. These pre-calculations became validation calculations of the NTDiv012 database. C.4.5.2. Fe-Zr-O liquidus and solidus measurements Pre-calculations on the Zr-O-Fe system, made in support of the SKODA tests (WP2 task 2-2) were performed with the SKODA “measured” compositions called “chemical analysis” (with a clear uncertainty about the oxygen concentration). Different databases, provided by Thermodata (NTDiv01 Tdbcr991 and Tdbcr992) or CEA (DPC/01), corresponding to different Zr-O-Fe models were used to calculate Tsol and Tliq . A good agreement between measurements and calculations is obtained for Tsol. For Tliq, depending on the database, measurements are 300 to 1300 K lower than calculations. NTDiv seems to be the best database but with still 300 K discrepancy . The visual Tliq measurement method consisting to observe the temperature at which the sample forms a droplet, is probably less sensitive than methods related to sign of the last crystal presence (as TDA or VPA), giving an order of magnitude, located between Tliq and Tsol. Calculations about the phase proportion evolution versus temperature showed that with the Thermodata database, in a very small range of temperature (~ 50K), 80% of the mixture is melted. This could be interpreted a Tliq with the visual method used by SKODA. According to calculations, the last phase to be dissolved in the liquid, is the zirconium rich solution phase (HCP) for NTDiv and DPC/01, or the ZrO2 phase for Tdbcr992. Furthermore for TDBCR992, depending of the oxygen content in the alloy, the primary crystallization phase is the zirconium rich solution phase (HCP) for a low oxygen content (~2at%) or the ZrO2 phase for higher oxygen content (~7.8at%). This is not the case for the other databases, whatever the oxygen content between 2 and 7.8at%. The real oxygen composition of the alloy is thus an important parameter . C.4.5.3 Fission Products release (Vercors tests) The aim of VERCORS is to quantify the release rate of FPs from pieces of irradiated fuel pins heated up to 2800°C. It is assumed in the calculations made with GEMINI2 that all the elements are oxidized. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 22 Moreover the quantity of oxygen necessary to form corresponding stable stoichiometry oxides (UO2, ZrO2, LaO, BaO, RuO2, SrO) is taken into account for the calculations. The composition of the gas is taken into account only to determine the pO. The equilibrium temperature was considered as the maximum temperature of the test (2300°C). The comparison between measured releases and the calculation results are summarized in Figure 14 A and 14 B with several databases. The release of baryum is important for both atmosphere. There is an agreement between calculation and measurement whatever the atmosphere is. The release of lanthanium is small for both atmosphere. There is an agreement between calculation and measurement whatever the atmosphere is. Calculated Ru releases in oxidizing atmosphere are very elevated compared to the measured releases. The discrepancy concerning the U release in oxidizing atmosphere has several origins: The thermodynamic data of the UO3 gaseous species are very uncertain and must be improved later on in the NUCLEA database. UO3 is taken into account in the databases since 1998 (TDBCR 981 & after), not in 1997 (TDBCR 971); UO2 is considered as stoichiometric in TDBCR 971 and 981; it is only since 1991 (TDBCR991 and after) that UO2+/- x is described. The presence of UO3 enhances significantly the U release, even in the database where the stoichiometry of UO2 is no described (TDBCR 971 & 981). The discrepancy concerning the Sr release in reducing atmosphere (Figure 14 A) has several main origins: The presence in the database of some Sr condensed phase like SrUO3 (TDBCR 991, 992, 001) or solid solution (Sr xBa 1-x)UO3 (NTDiv01 and 02), reduces strontium release by trapping some Sr in the condensed state but this is not enough. The calculated release is very sensitive to the oxygen quantity taken into account in the initial mass balance. The calculated release can be divided by 2 if the gas volume taken into account is limited to the plateau at 2300°C instead of the total volume of gas flowing over the pellets above 1300°C. C.4.5.4 Corium pool stratification involving Fe-O-U-Zr (+Cr, Ni) In 1976 Hofmann performed small scale experiments to study the melting behavior of fuel (UO2) with its clad (Zircaloy 4) and the structure (stainless steel). The AX1 (Table XII) test showed the presence of 2 immiscible liquids with the metallic one, iron rich also enriched in uranium and zirconium. This miscibility gap behavior in the liquid phase has also been experimentally observed in the U-O-Zr-Fe quaternary system in the Isabel 1 installation. Thermodynamic calculations were performed with NTDiv01 with theGemini2 code, the previous databases TDBCR971, 991, 992, 2000-1 with the GEMINI2 code, the 4 elements Fe-O-U-Zr DPC/01 database developed by CEA/DPC with the Thermocalc code. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 23 TDPC/01 and TDBCR97 calculate an important content of U in the metallic liquid phase. This is not the case for tdbcr991, 992, 2000-1 nor for NUCLEA. The discrepancy between measurement and calculation is more important for the metallic phase than the oxide one : in the metallic liquid Zr and O in DPC and Zr, Fe, O in TDBCR are not correctly calculated. Almost no experimental results exist in the metal-oxide part of the U-O-Zr-Fe system. It explains why there are so many differences between the DPC and Thermodata databases. This shows that the metallic description of these databases need to be improved. C.5 Influence of uncertainties (WP 4) The first step was devoted to the uncertainties still present in the nuclear thermodynamic data bases. Six cases (reactor case or R&D case) were chosen : a VULCANO spreading experiment (VE-U3), the TMI2 corium composition in reducing atmosphere, a typical EPR composition, the gaseous substances produced during MCCI, gaseous substances for in-vessel corium and U1-xZrxO2+y hyperstoichiometric compositions. Uncertainties on thermodynamic outputs (Tliq, Tsol, solid, liquid mass fractions during solidification, enthalpy) were assessed from the user’s point of view. Some general rules were drawn (Ref. [27] [28]) : the uncertainties on the thermodynamic outputs are decreasing with the latest versions of the databases all the thermodynamic outputs don’t have the same uncertainties ; the uncertainties on the enthalpy, Tliq and Tsol are low for the successive databases, the uncertainties of the composition of liquids are low, between Tsol and Tliq, the uncertainties on the nature and the proportions of the existing substances are increasing; the uncertainties on the nature and the quantities of the existing gaseous substances are increasing. It’s possible to define a general rule to qualify a thermodynamic calculation in relation with the quality of the binary systems that constitute the corium composition : if all the pseudo-binary systems that constitute the corium composition have rankings higher than ***, uncertainties on temperature will be less than 50K if one or more pseudo-binary systems constituting the corium have rankings equal to *, an uncertainty on the temperature of 100 K could be expected. In the U-Zr-O system, with the previous modeling, without experimental data, uncertainties for O/M>2.00 were important. The last modeling is now correct. Another part of the task was related to the relevance of subsystems for the nuclear thermodynamic databases (Ref. [29]). 290 were assessed . 12% of them are the most important. The main elements of the in-vessel corium (U, Zr, Fe, O) belong to the most relevant subsystems. The knowledge and modeling of these subsystems is good except for the subsystems with iron oxides : for the binary and the ternary systems including iron oxides, there’s a need of reliable thermodynamic data for the end-users of NTD. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 24 C.6 Coupling methodologies to S.A. codes (WP 5) C.6.1 Coupling methodology of thermodynamic databases to SA codes (CROCO and ICARE/CATHARE) (Task 5.1) Different strategies for coupling the thermo-chemical databases for corium with severe accident codes were investigated by IRSN. They can be broadly separated into two groups : either a direct coupling between a thermo-chemical code and the severe accident code, either ''tabulation'' strategies where the phase diagram is ''pre-calculated'' and implemented in the severe accident code. The thermo-chemical databases for corium contains the description of the Gibbs energy G, for all the phases able to appear in an accidental transient for a given number of elements. The Gibbs energy may exhibit a very complex form. Therefore the presence of metastable minimum is often encountered. The thermo-chemical codes on the market use different algorithms to solve the difficult problem of research of the stable equilibrium in a multicomponent and non ideal system. On the basis of this analysis, it is not reasonable to directly couple a thermochemical code with a severe accident code in order to treat the corium thermodynamics. C.6.1.1 Tabulation of the phase diagram The feasibility of an automatic tabulation of the complex phase diagrams and its implementation in severe accident codes was investigated. The main advantage of this approach is to prevent the numerical problems inherent to the determination of the stable equilibrium in a complex thermodynamic system. This automatic pre-calculation consists in a meshing of the concentration domain followed by the calculation, for each point of this meshing, of the thermodynamic properties, as a function of temperature. The implementation of this strategy of coupling by meshing of the phase diagram requires four separate steps : the meshing of the composition and temperature domain, the interface with the thermo-chemical code, the generation of the tabulation, the development of tools allowing to get for any composition and any temperature, the value of the thermodynamic properties. This was done for the U-Zr-Fe-O system. C.6.1.2 Interface with the thermochemical code The FORTRAN version of the thermo-chemical code GEMINI2 was modified to make it more readable and to reduce the time of each call. (A factor of 10 was gained ). With this improvement, the tabulations up to 7 elements could be performed in few days. C.6.1.3 Generation of the library, research and interpolation in the library For each node of the tabulation (x), GEMINI2 is called in order to compute the useful thermodynamic quantities : Tsol, Tliq, the temperature of apparition of the gas phase, the liquid fraction, the solid fraction, the gas fraction, the enthalpy, the composition of the solid phase, the composition of the liquid phase, the composition of the gas phase. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 25 For each node of the meshing (x), one stores the different thermodynamic properties (previously mentioned) in a file (the format of this file respecting the MDB requirements). All these files constitute the associated material library. With the concentrations meshing and the corresponding material library, it is possible to get the properties of any material by interpolation. For obvious reasons of computation time, it is excluded to do a loop over all the elements of the composition mesh. The retained solution is to research the closest mesh nodes of the point to interpolate and then to research, the good ''element'' over the elements associated with these closest points, that is to say, the elements containing one or more of these points. Once the element containing the M point determined, the thermodynamic properties at M, have to be interpolated over the nodes of the considered element. The interpolation is performed firstly in the temperature tabulation and after in the composition field. C.6.1.4 Applications The U-Zr-O phase diagram meshing has been restricted to the composition sub-domain included in the triangle the UO2-ZrO2-Zr triangle. This meshing contains 289 nodes. Some compositions have been chosen in order to validate the approach. As example, some reconstructions of different thermodynamic properties for the composition UO2-ZrO2-Zr = 11-1 in moles were calculated : the enthalpy, the liquid fraction, the liquid composition (Figure 15) and the solid composition, and compared with the GEMINI2-NUCLEA “referenced” results. A good agreement is obtained. The meshing of the quaternary phase diagram U-O-Zr-Fe (with 1576 nodes) has been also investigated and the “tabulation” approach remains feasible and suitable (Ref. [30]). C.6.1.5 Time consuming and coupling The generation of the tabulation of the different ternary phase diagrams, takes less than 1 hour, and for the quaternary phase diagrams, only a few hours. Phase diagrams with 6 or 7 elements should request a few days. In any case, it means that the tabulation can be easily and quickly re-generated with each new version of the database. For the quaternary phase diagram, the calculation of a thermodynamic property at a given composition and given temperature is 1000 times more rapid than a call to the GEMINI2 code. This time is reasonable, regarding the coupling with SA codes. The tabulation of the quaternary phase diagram occupies 22 Mbytes. Phase diagrams with 6 or 7 elements could occupy 1 Gbytes, which can be prohibitive. Further analysis must be performed for phase diagrams with such high number of elements. The implemented approach will be included in the MDB module of the ASTEC Code. C.6.2 Recalculation of TMI2 with MAAP4 and the database (Task 5.2) EDF uses the EPRI MAAP integral software in order to calculate SA sequences for PSA level 2. MAAP4 models thermal-hydraulics and FPs behaviour in the primary circuit, steam generators, containment, and auxiliary buildings. In this code is implemented the MATPRO thermo-chemical. The effect on plant applications of the new properties given by NUCLEA was investigated (Ref. [31]). FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 26 C.6.2.1 Thermo-chemistry of the U-Zr-O domain The general process of degradation is strongly governed by the formation of the U-Zr-O eutectic. The effect of the melting temperature of the U-Zr-O mixture can be investigated by the opportunity of two models implemented in MAAP4 . C.6.2.2 Recalculation of the U – Zr – O diagram using the NUCLEA database The two pseudo-ternary diagrams of U-Zr-O were recalculated using the NUCLEA database. Tliq and Tsol of the leading points of the diagrams were evaluated, postulating a similar shape between the leading points as in the previous MATPRO database. The differences between MATPRO and NUCLEA for Tliq are summarised in Table XIII. The calculations were done with the GEMINI Gibbs minimizer. For several points, temperatures are very close (less than 30 K, sometimes much less). There are some large differences such as Tliq of corium but this corium contains no Zirconium and is therefore mainly non prototypic. C.6.2.3 Recalculation of TMI-2 using the new Tliq and Tsol The new calculation, made with NUCLEA data for U-Zr-O was compared with a previous calculation of the TMI-2 sequence run for the CIT project (in the 4th Framework Program). The two main physical parameters investigated are the total mass of hydrogen produced during the accident (300 kg were produced before quenching and 150 kg after the quenching) and the mass of molten materials. With the initial reference calculation made with MATPRO, the total mass of molten materials is notably too low : 25 tons instead of 60 tons. With the new figures extracted as explained above from the U-Zr-O subsystem of the NUCLEA database, the calculated mass of molten materials is of the same order of magnitude (25 tons). The trend of the H2 production is also the same for both calculations. C.6.3 Simplification of the database and/or of equilibrium code and adaptation to SA codes (Task 5.3) Users are interested by simplifying the NTD-GEMINI2 global thermodynamic approach for specific applications, in order to decrease significantly the calculation time for one thermo-chemical calculation, and also to guarantee the convergence of their codes at 100 %, i.e. the robustness. Specific subsets may be extracted from the global NTD user’s request. In this respect the Fe-O-U-Zr database was assembled and delivered to IRSN, EDF, CEA. Concerning this « key » quaternary system, improvement works were documented : boiling points and vapour pressure of Fe, U and Z critical assessment of some systems Fe-U, U-Zr, O2U-O2Zr, shared between THERMODATA and AEA-T (Ref. [32]), improved version using a sublattice model to describe the UO2+x. The partial FeOUZr Thermodynamic Database for In- and Ex-Vessel applications, contains the 4 + 2 following elements : O-U-Zr-Fe + Ar-H, and includes in particular the 4 oxide system : UO2 -ZrO2 -FeO-Fe2O3. Hydrogen was added because it is a major component of the system, but the dissolution of hydrogen in condensed solid and liquid solutions is not taken into account at this time. The FeOUZr Thermodynamic Database can be considered as well validated. FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 27 C.7 Edition of the database All the binary systems and many ternaries were checked, and the data base as a whole too. The database for in- and ex-vessel applications was named “NUCLEA” A commercial agreement was signed between THERMODATA and AEA-T on the property rights. CONCLUSION As outcome of the extensive efforts, started in the 3rd Framework Programme for R&D of the European Commission, through the 4th and 5th FWP (the present ENTHALPY Project) , the NUCLEA database is now available. This tool is unique in the world to calculate the phase diagrams of most of the corium materials involved in severe accidents (Ref. [33]). This was reached by merging the two databases existing in Europe at the beginning of ENTHALPY, and by extending it to key new elements. The database was feed up with experimental results for many important systems that were unknown or badly known. The database was validated against many types of experiments. Methodologies were developed to link it with different SA codes in a fast and reliable way. The database is fully documented and commercially available; more information can be get on the web-site : http://thermodata.online.fr/nuclea/Nuclea03-1.htm The next step, beside the necessary updating of data, should be the introduction of the following new elements : Mo, Pu, Cs. The ENTHALPY partners thank the European Commission for its thorough scientific and financial support to the Project. REFERENCES [1] Chevalier P.Y., Fischer E., Assessment and assembling of the systems for the Nuclear Thermodynamic Database NTDiv01, ENTHALPY Report SAM-ENTHA(01) P003 (February 2001) [2] THERMODATA, NTDiv01 Nuclear Thermodynamic Database for In-Vessel applications, ENTHALPY report SAM-ENTHA(01) D001 (February 2001) [3] Mason, P.K. Mignanelli M.A, Extension of the oxide components of the nuclear thermodynamic database for in-vessel applications, ENTHALPY report SAMENTHA(01) P007 (November 2001) [4] Chevalier P.Y., Fischer E., Nuclear Thermodynamic Database for In-Vessels applications. Extension to B and C, ENTHALPY report SAM-ENTHA(02) P009 (February 2002) FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 28 [5] Chevalier P.Y., Fischer E., B – U and C – U : Thermodynamic assessment, ENTHALPY report SAM-ENTHA(02) P010 (February 2002) [6] THERMODATA, Nuclear Thermodynamic Database. 2nd version for In-Vessels applications, ENTHALPY report SAM-ENTHA(02) D002 (February 2002) [7] Mason P.K. and Mignanelli M.A., Extension of the oxide components of the nuclear thermodynamic database for ex-vessel applications, ENTHALPY report SAMENTHA(02) P013 (June 2002) [8] Chevalier P.Y., Fischer E., Progress in the thermodynamic modelling of the O-U binary system, ENTHALPY report SAM-ENTHA(02) P012 (May 2002) [9] Barrachin M., Mignanelli M., Chevalier P.Y., Comparison exercise between the thermo-chemical databases NUCLEA and MTOX regarding the pseudo-quaternary phase diagram FeO-Fe2O3-CaO-SiO2, ENTHALPY report SAM-ENTHA(03) P017 (March 2003) [10] Punni J.S., Mignanelli M.A., Determination of the Solidus and Liquidus temperatures of uranium-zirconium oxides, ENTHALPY report SAM-ENTHA(01) D004 (August 2001) [11] VrtílkováV., Novotný L., Belovsky L., Solidus temperatures in Zr-Fe-O and Zr-Cr-O systems, ENTHALPY report SAM-ENTHA(01) D005 (November 2001) [12] Vasáros L., Jákli Gy, Pintér A., Hózer Z., High Temperature Investigation of the Iron Oxide-B2O3 Systems, ENTHALPY report SAM-ENTHA(01) D008 (January 2001) [13] Vasáros L., Jákli Gy, Windberg P., Matus L., Nagy I., Pintér A., Hózer Z., Phase Transition Investigation in the ZrO2 -B2O3 system, ENTHALPY report SAMENTHA(01) D009 (January 2003) [14] Vasáros L., Jákli Gy, Hózer Z., Phase Transition Investigation in the UO2 -B2O3 system, ENTHALPY report SAM-ENTHA(01) D010 (January 2003) [15] Hellmann S., Lopukh D., Liquidus in subsystems UO2-ZrO2-(Al2O3-SiO2-CaO-FeOCr2O3-BaO), ENTHALPY report SAM-ENTHA(03) D011 (March 2003) [16] Duvigneaud P.H., Kitembo Mwamba, Phase relationships in the BaO-ZrO2-MoO3UO2 system, ENTHALPY report SAM-ENTHA(02) D013 (July 2002) [17] Bouchana, H., Cara J., Ronneau Cl., Behaviour of UO2-MoO2-BaO-ZrO2 at high temperature (element migration and release, influence of gases), ENTHALPY report SAM-ENTHA(01) P006 / SAM-ENTHA(01) D015 (June 2001) [18] Verwerft M., Vos B., Van den Berghe S., Review of the U-Zr-Mo-Ba-O quinary system, ENTHALPY report SAM-ENTHA(03) D016 (January 2003) FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 29 [19] Mason P.K., Mignanelli M.A., Comparison of the model for UO2+x in the NTD with empirical correlations and experimental data, ENTHALPY report SAM-ENTHA(02) D017 (August 2002) [20] Barrachin M., Minutes of the third progress meeting, Budapest 5-6 July 2001, ENTHALPY report SAM-ENTHA(01) M005 (July 2001) [21] Journeau Chr., Piluso P., Interpretation, with GEMINI2 and TDBCR992 of the corium cooling in the VULCANO test VE-U3, ENTHALPY report SAM ENTHA(00) P001 (August 2000) [22] Journeau Chr., Interpretation of the VULCANO VE-U7 data using GEMINI2 and the TDBRCR001 database, ENTHALPY report SAM-ENTHA(02) P016 (December 2002) [23] Piluso P., Validation of the European thermodynamic database with the experiments performed at NRI-REZ on the (U,Zr)O2 – Fe2O3 system, ENTHALPY report SAMENTHA(03) D020 (January 2003) [24] Cheynet B., Chaud P., Boiling points and vapour pressures of elements. Improvement from literature, ENTHALPY report SAM-ENTHA(02) D021 (February 2002) [25] Cheynet B., Chaud P., Thermodynamic properties of gases in the O-U system, ENTHALPY report SAM-ENTHA(03) D22 (March 2003) [26] Defoort Fr., Pre-calculations of UO2+x–ZrO2 (AEA-T) and Fe-Zr-O (SKODA) Tliq and Tsol measurement; Validation of the database based on global experiments (Vercors test and Hofmann tests), ENTHALPY report SAM-ENTHA(03) D023 (January 2003) [27] Piluso P., An evaluation of the uncertainties of the thermodynamic database, ENTHALPY report SAM-ENTHA(02) D024 (February 2002) [28] Piluso P., Consequences of the uncertainties in the database, ENTHALPY report SAM-ENTHA(03) D025 (March 2003) [29] Piluso P., Relevance of subsystems for the nuclear thermodynamic databases, ENTHALPY report SAM-ENTHA(02) P015 (September 2002) [30] Barrachin M., Jacq F., Coupling of the corium thermo-chemical database with the severe accident codes, ENTHALPY report SAM-ENTHA(03) D026 (January 2003) [31] Marguet S., Calculation of TMI-2 using new Liquidus and Solidus temperature of UZr-O, ENTHALPY report SAM-ENTHA(03) D027 (March 2003) [32] THERMODATA, FeOUZr Thermodynamic database, ENTHALPY report SAMENTHA(03) D028 (March 2003) [33] THERMODATA, NTD Nuclear Thermodynamic Database for In- and Ex-Vessel Applications, ENTHALPY report SAM-ENTHA(03) D003 (March 2003) FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 30 Table I : Summary of WPs, tasks and partners in the ENTHALPY project. WP WP 1 Task 1.1 & 1.2 WP 2 Task 2.1 Task 2.2 Task 2.3 Task 2.4 Task 2.5.1 Task 2.5.2.1 Task 2.5.2.2 Task 2.5.2.3 WP 3 Task 3.1 Task 3.2 Task 3.3 Task 3.4 Task 3.5 WP 4 WP 5 Task 5.1 Task 5.2 Task 5.3 WP 6 Description of WP and tasks Assembling & extension -Critical assessment ,assembling; extension Separate Effect Tests -Hyperstochiometry in UO2+x - ZrO2 -Tliq in Zr - Fe - O and Zr - Cr – O -Tliq in B2O3-FeOx-ZrO2-UO2 -Tliq in UO2-ZrO2-FeOx-CaO-Al2O3 -Tliq in UO2 – BaO -UO2-ZrO2-MoO2-BaO phase diagram -Release in ,, in T gradient -Comparison with damaged irrad. fuel pins Validation and improvement -From fuel experimental & empiric models -Based on ACE experiments -Based on VULCANO, COLIMA -Based on literature -Based on UO2+x-ZrO2 test , Vercors etc Influence of uncertainties Coupling methodologies to SA codes -To CROCO, ICARE/CATHARE -Recalculation of TMI1 with MAAP4 -Simplification of database & equilibr. code Edition of the database FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 Main partners THERMODATA , AEA-T AEA-T SKODA-UJP AEKI FRAMATOME ANP FRAMATOME ANP ULB UCL SCK.CEN-Mol AEA-T IRSN (ex-IPSN) CEA/DRN/DTP THERMODATA CEA-Grenoble CEA/DRN/DTP IRSN EdF IRSN THERMODATA January 2004 31 Table II : State of the validation of the binary systems N°NAME 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 Ag-Al Ag-B Ag-Ba Ag-C Ag-Ca Ag-Cr Ag-Fe Ag-In Ag-La Ag-Mg Ag-Ni Ag-O Ag-Ru Ag-Si Ag-Sr Ag-U Ag-Zr Al-B Al-Ba Al-C Al-Ca Al-Cr Al-Fe Al-In Al-La Al-Mg Al-Ni Al-O Al-Ru Al-Si Al-Sr Al-U Al-Zr B-Ba B-C B-Ca B-Cr B-Fe B-In B-La B-Mg B-Ni B-O B-Ru B-Si B-Sr B-U B-Zr Ba-C Ba-Ca Ba-Cr S QC O T T T T T T C T T T T T T T T T T T O O T O O T O O C T O T T O T T T T O T T O O T T T T T T T T T *** * *** ** ** * *** *** *** ** *** *** *** *** *** ** *** ** ** *** *** *** *** *** ** *** ** * ** *** ** ** * * ** * ** *** * ** *** *** * ** ** * ** *** * *** * N° NAME S QC N° NAME S QC 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 Ba-Fe Ba-In Ba-La Ba-Mg Ba-NIi Ba-O Ba-Ru Ba-Si Ba-Sr Ba-U Ba-Zr C-Ca C-Cr C-Fe C-In C-La C-Mg C-Ni C-O C-Ru C-Si C-Sr C-U C-Zr Ca-Cr Ca-Fe Ca-In Ca-La Ca-Mg Ca-Ni Ca-O Ca-Ru Ca-Si Ca-Sr Ca-U Ca-Zr Cr-Fe Cr-In Cr-La Cr-Mg Cr-Ni Cr-O Cr-Ru Cr-Si Cr-Sr Cr-U Cr-Zr Fe-In Fe-La Fe-Mg Fe-Ni C T C T T T C C C C C T O O T T T O T T O T T O T T T T O T T T T T T T O T T O O O T O T T T T T O O 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 Fe-O Fe-Ru Fe-Si Fe-Sr Fe-U Fe-Zr In-La In-Mg In-Ni In-O In-Ru In-Si In-Sr In-U In-Zr La-Mg La-Ni La-O La-Ru La-Si La-Sr La-U La-Zr Mg-Ni Mg-O Mg-Ru Mg-Si Mg-Sr Mg-U Mg-Zr Ni-O Ni-Ru Ni-Si Ni-Sr Ni-U Ni-Zr O-Ru O-Si O-Sr O-U O-Zr Ru-Si Ru-Sr Ru-U Ru-Zr Si-Sr Si-U Si-Zr Sr-U Sr-Zr U-Zr T C O C C T T T T T T T T T T T T T T T C T C O T T O T T T O T O T T T T C T T T T C C C C C O C C C FINAL SUMMARY REPORT "ENTHALPY" ** *** ** ** ** ** * ** ** * * * *** *** * ** * *** * *** *** * **** *** * * ** ** *** ** * * ** *** * * **** * ** * **** *** ** *** * *** ** *** *** ** **** SAM(ENTHA)04-P019 January 2004 32 *** *** *** * ** ** *** *** **** ** * *** *** * * ** *** * ** ** * *** * ** * * *** ** * ** *** *** *** ** ** *** * ** * *** *** ** * *** *** *** ** ** * * *** Table III : State of the validation of the pseudo-binary systems N° NAME S QC N° NAME S QC 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 Al2O3-B2O3 Al2O3-BaO Al2O3-CaO Al2O3-Cr2O3 Al2O3-FeO Al2O3-Fe2O3 Al2O3-In2O3 Al2O3-La2O3 Al2O3-MgO Al2O3-NiO Al2O3-SrO Al2O3-SiO2 Al2O3-UO2 Al2O3-ZrO2 B2O3-BaO B2O3-CaO B2O3-Cr2O3 B2O3-FeO B2O3-Fe2O3 B2O3-In2O3 B2O3-La2O3 B2O3-MgO B2O3-NiO B2O3-SrO B2O3-SiO2 B2O3-UO2 B2O3-ZrO2 BaO-CaO BaO-Cr2O3 BaO-FeO BaO-Fe2O3 BaO-In2O3 BaO-La2O3 BaO-MgO BaO-NiO A C O T T C T C C T T C C C A A A A A A A A A A A A A T T C T T C T 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 BaO-SrO BaO-SiO2 BaO-UO2 BaO-ZrO2 CaO-Cr2O3 CaO-FeO CaO-Fe2O3 CaO-In2O3 CaO-La2O3 CaO-MgO CaO-NiO CaO-SrO CaO-SaO2 CaO-UO2 CaO-ZrO2 Cr2O3-FeO Cr2O3-Fe2O3 Cr2O3-In2O3 Cr2O3-La2O3 Cr2O3-MgO Cr2O3-NiO Cr2O3-SrO Cr2O3-SiO2 Cr2O3-UO2 Cr2O3-ZrO2 FeO-Fe2O3 FeO-In2O3 FeO-La2O3 FeO-MgO FeO-NiO FeO-SrO FeO-SiO2 FeO-UO2 FeO-ZrO2 Fe2O3-In2O3 C T T C T O C T C O T T O C C T T T T T T T T T T T T T T T T T C T T ** *** *** ** *** ** * .** ** .* ** ** *** *** ** ** ** ** ** ** ** ** ** ** ** ** ** * * *** ** ** ** ** ** ** ** *** ** * ** * *** *** ** ** *** ** *** ** ** * *** ** * * * * * *** * * * ** ** ** ** ** * N° 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 NAME S QC Fe2O3-La2O3 Fe2O3-MgO Fe2O3-NiO Fe2O3-SrO Fe2O3-SiO2 Fe2O3-UO2 Fe2O3-ZrO2 In2O3-O3LA2 In2O3-MgO In2O3-NiO In2O3-SrO In2O3-SiO2 In2O3-UO2 In2O3-ZrO2 La2O3-MgO La2O3-NiO La2O3-SrO La2O3-SiO2 La2O3-UO2 La2O3-ZrO2 MgO-NiO MgO-SrO MgO-SiO2 MgO-UO2 MgO-ZrO2 NiO-SrO NiO-SiO2 NiO-UO2 NiO-ZrO2 SrO-SiO2 SrO-UO2 SrO-ZrO2 SiO2-UO2 SiO2-ZrO2 UO2-ZrO2 C C T C C C C T T T T T T T C T ** C C C T C O C C T T T T C T C C C C ** ** ** ** * * * * * * * * * * *** * ** ** *** ** *** *** ** *** * * * * ** ** *** *** *** *** Table IV : State of the validation of the ternary systems N° 1 2 3 4 5 6 7 NAME S QC N° NAME S QC N° NAME S QC B-C-Fe B-C-U B-C-Zr B-Fe-U B-Fe-Zr C-Cr-Fe C-Cr-Ni T ** T ** T ** T ** T * O *** O *** 8 9 10 11 12 13 14 C-Fe-Ni C-O-U C-O-Zr C-U-Zr Cr-Fe-Ni Cr-Fe-O Cr-Fe-Zr O **** T ** T ** T * T ** T ** T ** 15 16 17 18 19 20 Cr-Ni-O Fe-Ni-O Fe-O-U Fe-O-Zr Fe-U-Zr O-U-Zr T ** T ** T ** T ** T ** T *** FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 33 Table V : State of validation of the pseudo-ternary systems N° 1 2 3 4 5 6 7 NAME S QC N° NAME Al2O3-CaO-FeO Al2O3-CaO-Fe2O3 Al2O3-CaO-SiO2 Al2O3-SiO2-UO2 Al2O3-SiO2-ZrO2 Al2O3-UO2-ZrO2 SiO2-UO2-ZrO2 * * C *** T ** C ** C ** C ** 8 9 10 11 12 13 14 Al2O3-FeO-Fe2O3 Al2O3-FeO-SiO2 Al2O3-Fe2O3-SiO2 CaO--FeO-Fe2O3 CaO--FeO-SiO2 CaO-Fe2O3-SiO2 FeO-Fe2O3-SiO2 - NOTE : N° NAME S QC S QC * * * * * * * N° NAME S QC 15 16 17 18 19 20 21 Al2O3-B2O3-CaO Al2O3-B2O3-SiO2 B2O3-CaO-SiO2 B2O3-FeO-Fe2O3 Al2O3-B2O3-MgO B2O3-CaO-MgO B2O3-MgO-SiO2 A A A A A A A Order number Name of the system Source : T = Thermodata, A = AEA-T, O = open literature, C = common (T-AEA-T) Quality criterion (*, **, ***, ****). Table VI : Solidus and liquidus temperatures for UO2+x-ZrO2 mixtures Sample mixture (80/20 wt%) UO2.00-ZrO2 UO2.08-ZrO2 UO2.15-ZrO2 Composition U0.65Zr0.35O2.000 U0.65Zr0.35O2.052 U0.65Zr0.35O2.098 Solidus (C) 2590 2550 2482 Liquidus (C) 2678 2638 2561 Table VII : Liquidus temperatures measured in the B2O3/ FeOx/ ZrO2/ UO2 systems B2O3–Fe2O3 Fe2O3 Tliq (wt %) (°C) 5.0 885 7.5 1010 10.0 1065 15.0 1130 18.0 1165 25.0 1225 37.5 1320 B2O3–ZrO2 ZrO2 Tliq (wt %) (°C) 2.5 1130 5.0 1300 1800 6.5 0.5 2000 13.51.0 FINAL SUMMARY REPORT "ENTHALPY" B2O3–UO2 UO2 Tliq (wt %) (°C) 2.5 1130 5.0 1300 7.5 1330 10.0 1460 SAM(ENTHA)04-P019 January 2004 34 ** ** ** ** ** ** ** Table VIII : Compositions, Tliq and Tsol of the ex-vessel mixtures tested Sample 1 2 3 4 5 6 7 8 9 10 Compositions (wt%) UO2 43.3 42.1 33.8 37.9 33.6 41.7 41.4 0.3 61.9 3.5 24.2 ZrO2 17.8 13.9 14.5 13.5 13.5 16.4 15.5 39.5 14.9 9.5 7.9 Others 28.9 FeO – 10.0 Cr2O3 31.4 FeO – 12.6 Cr2O3 30.1 FeO - 14.1 SiO2 - 7.5 Cr2O3 16.9 FeO - 1.1 SiO2 – 12 Cr2O3 - 7.6 CaO 26.4 CaO - 26.5 Al2O3 41.5 FeO – 0.4 Al2O3 12FeO -13.6SiO2 - 4.2Cr2O3 -7Al2O3 - 6.3CaO 28.8FeO-12SiO2-3.6Cr2O3-13.1Al2O3-2.7CaO 23.2 CaO 33.5 CaO - 25.5 SiO2 33.8 CaO - 9.1 FeO - 25 SiO2 Experimental results Tliq (°C) Tsol (°C) 1710-1760 1600-1640 1660-1700 1770 1800-1860 1800-1860 1800-1880 1890-1980 1800-1860 1800-1880 1420 1360-1400 1390±30 1340 1350 1250 1320 1860-1870 1460-1520 1240-1360 Table IX : Compositions and test conditions of heated samples from the U-Zr-Ba-Mo-O system Oven ΔG(O2) Samples & test T (°C) kJ/mol conditions 1500 ~ - 25 A : 30 min/1500°C D : 30 min/1500°C F : 3 min/1500°C Nitrogen N57 1500 ~ - 250 A : 60 min/1500°C D : 60 min/1500°C F : 60 min/1500°C Nitrogen N57 1500 ~ - 450 A : 60 min/1500°C + 20 ppm H2 D : 60 min/1500°C F : 60 min/1500°C Ar N50 2000 ~ A : 30min/ 2000°C (AAS oven) 1000 D : 30min/ 2000°C F : 30 min/ 2000°C Emission gaz Air Results : Compositions of pellets after heating A : UO2,U3O8,BaMoO4,MoO3, traces ZrO2 D : UO2,U3O8,Ba,BaO,BaMo4O13,traces ZrO2 F : UO2,U3O8, U4Zr11O32, traces ZrO2 A : UO2,25, traces ZrO2 D:UO2+x, Ba, traces ZrO2 F : U3O7, traces ZrO2 A : UO2, Mo, BaMoO4, BaZrO3 D : UO2,BaZrO3, Ba2UO2O7 F : UO2, BaZrO3, ZrO2,Ba3Mo18O28 A : UO2,25, Mo, U, Zr, Ba D:UO2,U,Zr,Ba,trace Mo F : unknown cubic U/Zr? Table X : ORIGEN-2 calculation of production rates of fission products Zr, Mo and Ba expressed per GWd/tHM, for BWR fuel of a BU of 56GWd/tHM. Zr UO2 (U,Pu)O2 113 80 Mo Wt ppm produced per GWd/tHM 105 97 FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 Ba 48 45 January 2004 35 Table XI : Tboiling of 11 elements of the database after literature overview Boiling temperatures (in Kelvin) of 11 elements of the database Element Hulgren Kubashewski Janaf TCras SGTE This work Ag 2430 2432 2437 2430 2435 Ba 2210 2167 2118 2210 2080 Cr 2953 2952 2952 2953 2768 Fe 3122 3133 3133 3125 3122 3163 In 2290 2343 2295 2290 La 3713 3730 3686 3713 Ni 3118 3169 3156 3130 3118 3366 Ru 4633 4612 4633 Sr 1635 1635 1685 1635 1656 U 4457 4440 4470 4457 4149 Zr 4700 4630 4702 4630 4700 4886 max 5 130 185 208 248 29 308 256 Table XII : Global compositions of Isabel 1 and Hofmann AX1 tests Fe Global composition in at% U Zr ISABEL 1 [GUÉNEAU, 1999] 50 17 18 15 HOFMANN AX1 [HOFMANN, 1976] C14 U/ZR=1.2 20.3 15.3 19.4 45 (+Cr+Ni for Hofmann) O Table XIII : Liquidus temperatures as calculated with MATPRO and NUCLEA (limited to the elements U, Zr, O) and the GEMINI2 Gibbs minimiser Point MAAP XO XU XZr TMAAP (K) TNUCLEA (K) a b c d e f g h k l m n p q r 0.666 0.6666 0.6666 0.59 0.59 0.46 0.44 0.40 0.25 0.40 0.10 0 0 0.07 0.6666 0.3333 0.1666 0 0.41 0.27 0.54 0.13 0.09 0 0 0 0 1 0.93 0.13 0 0.1666 0.3333 0 0.14 0 0.43 0.51 0.75 0.60 0.90 1 0 0 0.2033 3113 2800 2911 2740 2673 2740 2673 2338 2403 2338 2243 2125 1406 1406 2850 3119 2849 2984 2759 2652 2766 2458 2357 2424 2408 2254 2127 1407 2899 2851 FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 36 Figure 1 : Calculated phase diagrams for the Fe2O3 -B2O3 with a few experimental points Figure 2 : Assessment of the O-U phase diagram (spec. in the UO2+/-x region) FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 37 Cu 1100 1500 600 800 1000 500 Ag 950 450 900 400 850 A 800 Solidus-A Solidus-B Liquidus-A Liquidus-B HV-10 B a) C D E F Zr-Fe-O Temperature [ C] 550 Hardness HV-10 Temperature [ C] 1050 1400 700 1300 600 1200 500 1100 400 Cu 1000 350 900 300 800 300 Solidus-A Liquidus-A HV-10 b) Solidus-B Liquidus-B 200 Zr-Cr-O A B C 0 0.2 0.4 D E 0.6 0.8 Hardness HV-10 Ni 100 1 0.2 0.4 0.6 0.8 1.2 Fig. 10 0: Solidus and liquidus1 temperatures of the tested alloys versus nominal Nominal oxygen content Nominal oxygen content ] oxygen content. The[wt% dashed ellipse marks the required alloy.[wt% ] a) Zr-Fe-O b) Zr-Cr-O Figure 3 : Measured Tsol & Tliq and hardness versus nominal oxygen content of Zr-Fe-O & Zr-Cr-O alloys 1600 1800 1500 Liq. Fe2O3 + Liq. B2O3 1400 1700 1600 1300 1500 1200 1100 1400 Solid Fe2O3 + Liq. B2O3 o T, C 1200 900 Fe2O3 + Fe3BO6 700 Solid Fe3BO6 + Solid FeBO3 Solid Fe2O3 + Solid FeBO3 600 1100 1000 Solid FeBO3 + Liq. B2O3 FeBO3 Fe3BO6 800 Solid Fe3BO6 +Liq. B2O3 T, K 1300 1000 900 500 800 Sintered Fe2O3 + B2O3 700 400 Solid Fe2O3 +Solid B2O3 600 300 0 10 20 30 40 50 60 70 80 90 100 B2O3, wt % Experimental, Interpolated, Lit. [4] Figure 4 : Phase diagram for the Fe2O3 – B2O3 system with the 8 experimental points (red circles) gained in the ENTHALPY project FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 38 Figure 5 : Experimental ZrO2-B2O3 (left;B2O3 side ) & UO2–B2O3 (right) phase diagram. The red circles are experimental points Liquidustemperaturverlauf bei der Mischung einer Schmelze aus 75 w% UO2 und 25 w% ZrO2 mit den 3 definierten Portlandzementtypen Viskositätsverlauf beim Aufschmelzen von PZ-Betonen 3000 1000000 SI-PZ 15 - liquidus FESI-PZ 15 - liquidus 2800 100000 EO-PZ 15 - liquidus 2600 10000 Viskosität [Pas] Temperatur [K] 2400 2200 1000 100 2000 10 1800 1 1600 1400 0 10 20 30 40 50 Anteil Beton [Gew%] Calculated liquidus temperatures for dissolving different concretes in 75% UO2-25% ZrO2 60 0,1 1000 SI-PZ15 FESI-PZ15 EO-PZ15 1100 1200 1300 1400 1500 1600 1700 Temperatur [°C] Calculated viscosities of different concretes with 15% Portland cement upon heating Figure 6 : Examples of benefits from the knowledge of Tsol and Tliq: calculation of the liquidus of different mixtures of ex-vessel corium mixtures (left) and of the viscosities of different concretes FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 39 B a O 0 1 0 0 K = 2 4 ; 1 6 0 0 ° C 1 0D B a U O + U O 3 s s 2 s s 9 0 2 0 B a M o O 8 0 3 6 C 3 0 B a Z r O 2 4 7 0 B a U O + M o + U O 3 s s 2 s s B a Z r O 4 0 3 2 7 B a Z r O 35 0 B a Z r O + U O 3 s s 2 s s 6 0 B m B a M o O 3 5 0 B G B m z 6 0 4 0 A 7 0 8 0 3 0 B a Z r O + M o + U O 3 s s 2 s s 2 0 Z r O + U O 2 s s 2 s s 9 0 1 0 0 0 Z r O 2 1 0 M o + U O 2 s s F B a Z r O + Z r O + M o + U O 3 s s 2 s s 2 s s E 0 1 02 03 04 05 06 07 08 09 01 0 0M o O ( x = 0 ) x B a O 0 1 0 0 K = 1 7 ; 1 6 0 0 ° C 1 0D 9 0 ( U , B a ) O + U O x 2 s s 2 0 B a Z r O 2 4 3 0 B a Z r O 4 0 3 2 7 B a Z r O + ( U , B a ) O + U O 3 s s x 2 s s B a Z r O 0 G 35 B a Z r O + U O 3 s s 2 s s 6 0 B a M o O + B a Z r O + ( U , B a ) O + U O 4 s s 3 s s x 2 s s B a M o O 8 0 3 6 C 7 0 B a M o O 2 5 6 0 B B m B a M o O 4 5 0 B m z A 3 0 B a M o O + M o O + U O 4 s s 2 s s 2 s s 8 0 2 0 B a M o O + Z r O + U O 4 s s 2 s s 2 s s 9 0 F 0 Z r O 2 B a M o O + U O 4 s s 2 s s 4 0 B a M o O 2 7 7 0 1 0 0 B a M o O + ( U , B a ) O + U O 4 s s x 2 s s 1 0 E 0 1 02 03 04 05 06 07 08 09 01 0 0M o O ( x = 2 ) Z r ( M o O ) 4 2 Z r O + U O 2 s s 2 s sB a M o O + Z r O + M o O + U O 4 s s 2 s s 2 s s 2 s s x M o O + U O 2 s s 2 s s b) Figure 6 : UO2 80wt% cut in the pyramid of the pseudoquaternary UO2-BaO-ZrO2MoOx system at 1600°C under pO2 = 10–k atm with a) k=16 (~-580kJ/mol) i.e. in reducing conditions b) k=9 to 5 i.e. in oxidizing conditions Phases stability domains relative to the UO2 80wt% matrix (in red) show substantial differences with respect to equilibrium phases evidenced at 1400°C (a) and 1600°C (b) in ternary BaO-ZrO2-MoOx system (in black dashed lines). FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 40 Mo Ba Cold Hot zone Zr U 50 µm Figure 8 : Mapping of the pellet A as a function of temperature Silica in liquid phase Scheil Gulliver TDBCR981 Equilibrium TDBCR981 Scheil Gulliver TDBCR 992 Equilibrium TDBCR 992 %mol SiO2 in liquid phase 50% 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% 1200 1400 1600 1800 2000 2200 2400 2600 2800 Temperature K Figure 9 : Evolution of the silica fraction in the liquid phase using the two versions of the database for equilibrium or Scheil-Gulliver cooling below 2270 K (VULCANO-VE-U3 spreading experiment) FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 41 Figure 10 : Proposed miscibility gap in the pseudo-ternary system ZrO2-SiO2-FeOx UO2(G) 4 2 0 logP(atm) -2 -4 -6 -8 -10 -12 0,0001 Szwarc 68 UO1.94 DeMaria 60 Ohse 66 (Ptot) Ohse 78 (Ptot) Ackerman 56 Babelot 86 Hoch 87 Benezech 74 : UO1.953 Long 80 Pattoret 68 Ackermann 69 Green 81 NTDiv00 Winslow 75 This work 0,0002 0,0003 0,0004 0,0005 0,0006 0,0007 1/T(K) Figure 11 : Ratio of the partial pressure of gaseous UO2 over condensed UO2 : comparison calculations vs experiments FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 42 In red : UO (G) In blue : UO2 (G) In pink : U (G) In green : UO3 (G) In turquoise : O (G) Figure 12 : Speciation of the gas over UO2 Calculation with the assessed data : T = 2500K. Figure 13A : Solidus temperature versus hyper- Figure 13B : Liquidus temperature versus hyperstoichiometry x : calculations with 2 versions of stoichiometry x : calculations with 2 versions of the database and measurements the database and measurements FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 43 Figure 13 : Solidus (left) and Liquidus (right) temperature versus hyper-stoichiometry x : calculations with 2 versions of the database and measurements FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 44 70 60 50 40 30 20 10 ea su re td bc r9 71 Td bc r9 81 Td bi vh 99 1 Td bi v9 91 Td bc r9 92 td bi v0 01 N td iv 00 1 nt di v0 21 0 VERCORS 4 90 80 BA 70 SR 60 RU 50 U 40 LA 30 ZR 20 U BA RU LA SR ZR 10 0 su re Td bc r9 71 Td bc r9 81 TD BI Vh 99 1 TD BI V9 91 td bi v9 92 TD BI V0 01 N TD iv 01 N TD iv 02 1 80 FP released in Vercors 5 (oxidizing) ea (FP in the gas phase) / (FP initial) 90 100 m FP released in Vercors 4 (reducing) M (FP in the gas phase) / (FP initial) 100 VERCORS 5 Figure 14 : Measured and calculated FP release in VERCORS 4 (left) and VERCORS 5 (right) Titre: Auteur: Aperç u: Cette image EPS n'a pas été enregis trée avec un aperç u intégré. Commentaires: Cette image EPS peut être imprimée s ur une imprimante PostScript mais pas s ur un autre type d'imprimante. Figure 15 : Liquid composition reconstruction from the tabulation versus GEMINI2NUCLEA results [Molar composition :(ZrO2, UO2, Zr)=(1,1,1)] FINAL SUMMARY REPORT "ENTHALPY" SAM(ENTHA)04-P019 January 2004 45