precipitation
advertisement

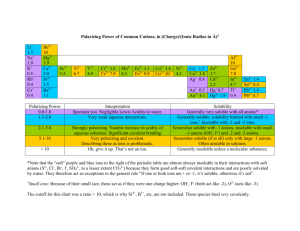
Precipitation Students will observe precipitation reactions among various ions and develop general solubility rules. (20 min) INTRODUCTION Objectives Students will design an experimental procedure. Students will be able to identify precipitation reactions. Students will draw conclusions based on observations. Students will develop general rules for solubility based on charge. Misconceptions There are never exceptions to rules. Student Difficulties Distinguishing between precipitation reactions and color changes. MATERIALS & METHODS Prerequisites Ions, chemical equations Equipment (Suggested Hardware & Software) Clear well plates nitrate solutions of the following (all 0.1 M solutions in dropper bottles): NH4+, K+, Ca2+, Sr2+, Mg2+, Al3+, Fe3+ sodium solutions of the following (all 0.1 M solutions in dropper bottles): Cl-, ClO4-, OH-, CO32-, SO42-, PO43- Activity Task Reason Notes Demo of a ppt reaction on an 1 overhead, e.g. AgNO3 + NaCl or Pb(NO3)2 + NaI Grabs attention and shows Shadows of the solid will that a solid can form when be visible on overhead two liquids are mixed. projector Give students a list of the chemical solutions and ask them 2 to design a way to logically and efficiently test for precipitates when two solutions are mixed. To have students design their own experimental procedure. A table may work best. Have students collect the data. 3 Only three drops of a solution should be used for each trial. Students will identify the combinations that yield precipitants. Placing clear wellplates on a black surface may help students to see the ppts better. Have students draw conclusions from their observations by 4 comparing the solutions that did or did not give precipitates. Students will see that certain ions form more ppts than others do. Some students may try to be more specific. Develop the solubility rules during a series of questions about 5 the students’ observations. See questions in Activity Notes Brings together the concept and students see that they can draw conclusions from simple observations. Draw the bridge between micro and macroscopic properties when discussing formation of precipitants. Have students develop a one sentence rule about solubility To develop a general rule based on charge. 6 DISCUSSION Activity Notes Previously, the dissolution of salt, or solution chemistry has been reviewed. Although, you have seen that many salts dissolve in water, there are many more which are not soluble. As a consequence, when two or more soluble compounds are mixed in water, a new insoluble substance may form. This insoluble substance is called a precipitant and this type of reaction is called a precipitation reaction. For example, when solutions of AgNO3 and Na3PO4 are mixed the following occurs: Ag1+ (aq) + NO31- (aq) + Na1+ (aq) + PO43- (aq) --> Na1+ (aq) + NO31- (aq) + Ag3PO4 (s) Ag3PO4 is an insoluble compound, a precipitant. Na1+ and NO31- are called spectator ions because they do not participate in the reaction. Therefore, a net equation can be written: Ag1+ (aq) + PO43- (aq) --> Ag3PO4 (s) In solution, ions of like charge will repel one another, while ions of opposite charge will attract one another. If the enthalpy of solvation is not enough to overcome solute enthalpy, then a precipitate may form. However, enthalpy of solvation is difficult to predict. Let’s consider Coulomb’s law F = q1q2/Er2 where F is the force of attraction for oppositely charged particles and the force of repulsion for like charged particles, q1 and q2 are the charges of interacting ions, r is the distance separating the charged particles, and E is the dielectric constant and is related to the polarity of a solvent. When opposite charges come together the magnitude of the charges determines the force of attraction between them. Therefore, compounds containing highly charged ions tend to be less soluble than compounds containing +1 and –1 ions. An example table and results for the precipitants formed in the probe is provided below. These results lend themselves well to the development of the solubility rules. See the questions provided below. Questions 1. What can be concluded about compounds containing ammonium ions? -they tend to be soluble 2. Did you find any exceptions to this observation? -No 3. What can be concluded about compounds containing potassium ions? -they tend to be soluble 4. Did you find any exceptions to this observation? -No 5. What about the perchlorate ions? -they tend to be solube 6. What about hydroxides? -they tend to be insoluble 7. What about carbonates? -they tend to be insoluble 8. What about sulfates? -they tend to be soluble, but there are exceptions 9. What about phosphates? -they tend to be insoluble 10. Separate the ions used in the lab based on their general trend of being soluble or insoluble. 11. What do the soluble ions have in common? -most of the soluble compounds have lower charges of (+/-)1 12. What do the insoluble ion have in common? -most of the insoluble compounds have charges of +/-2 and +/- 3. 13. Based on the general trends that you have observed, what would be one rule of thumb for determining solubility? -Higher charged ions tend to form insoluble compounds, while lower charged ions remain soluble Further questions: What rule would seem to be more important than the rest based on your observations? -Rule 1 because no precipitates ever formed with NH41+ or K1+ Why did we not see a precipitate when Ca2+ and SO42- were mixed? -CaSO4 is sometimes listed as soluble or slightly soluble rather than insoluble. It actually depends on the concentration of the ions. The CRC Handbook lists the Ksp of CaSO4 as 2.45 x 10-5, which is close to the solubility limit. Group Problem A grandmother lived in a rural area of the country noted for its limestone deposits (limestone is CaCO3). She often commented about the "hard" water" that she got from her well as a result of the limestone (hard water contains the positive ions Ca2+, Mg2+, and Fe2+.) She suffered from the swollen ankles and would often soak her feet in Epsom salts (Epsom salts contain MgSO4). One day her granddaughter came to visit and her. The grandmother asked the granddaughter to fix a solution of soaking salts so she could soak her swollen feet. The granddaughter was quick to help her grandmother out, but the granddaughter mistakenly poured NaCl in the pan of water assuming that was the "soaking salts" her grandmother requested. After 10 minutes the grandmother was ready for the soaking, but as soon as she saw the solution in the pan she knew her granddaughter had made a mistake. Since Epsom salts and table salt are both soluble in water, how did the grandmother know? (obtained from Kay Sandberg, NCSU) Related Activities Salt Solution (Students will see a microscopic view of how salt is dissolved by water.) Microscopic View (Students will classify particulate drawings based on microscopic properties.) REFERENCES 1. North Carolina State University CH 102 Laboratory Manual Department of Chemistry, Fall 2000, 43-46. 2. Wertz, Dennis W. Chemistry: A Molecular Science NCSU. Patterson Jones Interactive: 1999, page 10-12 – 10-13.