Overview
advertisement
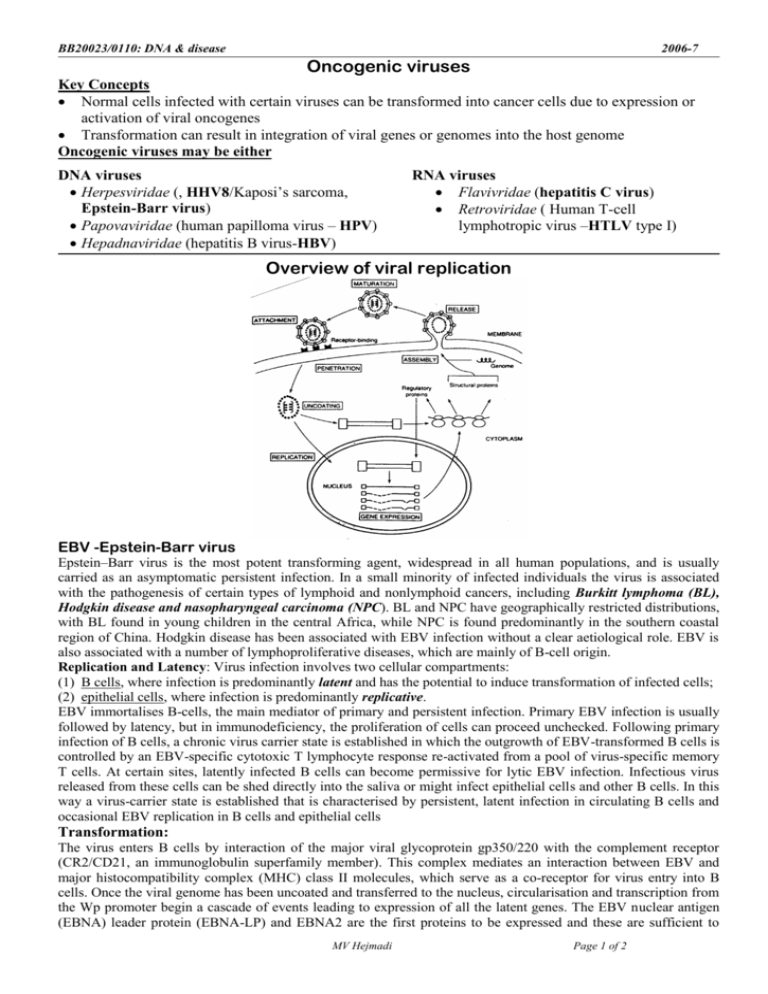
BB20023/0110: DNA & disease 2006-7 Oncogenic viruses Key Concepts Normal cells infected with certain viruses can be transformed into cancer cells due to expression or activation of viral oncogenes Transformation can result in integration of viral genes or genomes into the host genome Oncogenic viruses may be either DNA viruses Herpesviridae (, HHV8/Kaposi’s sarcoma, Epstein-Barr virus) Papovaviridae (human papilloma virus – HPV) Hepadnaviridae (hepatitis B virus-HBV) RNA viruses Flavivridae (hepatitis C virus) Retroviridae ( Human T-cell lymphotropic virus –HTLV type I) Overview of viral replication EBV -Epstein-Barr virus Epstein–Barr virus is the most potent transforming agent, widespread in all human populations, and is usually carried as an asymptomatic persistent infection. In a small minority of infected individuals the virus is associated with the pathogenesis of certain types of lymphoid and nonlymphoid cancers, including Burkitt lymphoma (BL), Hodgkin disease and nasopharyngeal carcinoma (NPC). BL and NPC have geographically restricted distributions, with BL found in young children in the central Africa, while NPC is found predominantly in the southern coastal region of China. Hodgkin disease has been associated with EBV infection without a clear aetiological role. EBV is also associated with a number of lymphoproliferative diseases, which are mainly of B-cell origin. Replication and Latency: Virus infection involves two cellular compartments: (1) B cells, where infection is predominantly latent and has the potential to induce transformation of infected cells; (2) epithelial cells, where infection is predominantly replicative. EBV immortalises B-cells, the main mediator of primary and persistent infection. Primary EBV infection is usually followed by latency, but in immunodeficiency, the proliferation of cells can proceed unchecked. Following primary infection of B cells, a chronic virus carrier state is established in which the outgrowth of EBV-transformed B cells is controlled by an EBV-specific cytotoxic T lymphocyte response re-activated from a pool of virus-specific memory T cells. At certain sites, latently infected B cells can become permissive for lytic EBV infection. Infectious virus released from these cells can be shed directly into the saliva or might infect epithelial cells and other B cells. In this way a virus-carrier state is established that is characterised by persistent, latent infection in circulating B cells and occasional EBV replication in B cells and epithelial cells Transformation: The virus enters B cells by interaction of the major viral glycoprotein gp350/220 with the complement receptor (CR2/CD21, an immunoglobulin superfamily member). This complex mediates an interaction between EBV and major histocompatibility complex (MHC) class II molecules, which serve as a co-receptor for virus entry into B cells. Once the viral genome has been uncoated and transferred to the nucleus, circularisation and transcription from the Wp promoter begin a cascade of events leading to expression of all the latent genes. The EBV nuclear antigen (EBNA) leader protein (EBNA-LP) and EBNA2 are the first proteins to be expressed and these are sufficient to MV Hejmadi Page 1 of 2 BB20023/BB20110: DNA: making, breaking & disease MV Hejmadi 2006-7 advance the cells to early G1 phase of the cell cycle. The EBV-infected (EBV+) cells begin to proliferate in a manner that depends on high cell density and on the autocrine production of cytokines that promote B-cell growth. Later, the EBV+ cells evolve into cells that grow more rapidly and are less dependent on autocrine growth mechanisms. Following infection in vivo, the virus establishes a latent infection in memory B cells and this is characterised by the limited expression of a subset of virus latent genes. Putative in vivo interactions between Epstein–Barr virus and host cells. a | Primary infection. Incoming virus establishes a primary focus of lytic replication in the oropharynx, after which the virus spreads throughout the lymphoid tissues as a latent (latency III) growthtransforming infection of B cells. Many of these proliferating cells are removed by the emerging latentantigen-specific primary-T-cell response, but some escape by downregulating antigen expression and establishing a stable reservoir of resting viral-genome-positive memory B cells, in which viral antigen expression is mostly suppressed (latency 0). Different views of these events are shown. One view envisages infection of pre-existing memory cells as a direct route into memory; this is consistent with the above observations on IM tonsils, although it still leaves unexplained the apparent disappearance of the infected naive cell population. b| Persistent infection. The reservoir of EBV-infected memory B cells becomes subject to the physiological controls governing memory-B-cell migration and differentiation as a whole. Occasionally, these EBVinfected cells might be recruited into germinal-centre reactions, entailing the activation of different latency programmes, after which they might either re-enter the reservoir as memory cells or commit to plasma-cell differentiation — possibly moving to mucosal sites in the oropharynx and, in the process, activating the viral lytic cycle. Virions produced at these sites might initiate foci of lytic replication in permissive epithelial cells, allowing low-level shedding of infectious virus in the oropharynx, and might also initiate new growth-transforming latency III infections of naive and/or memory B cells; these new infections might possibly replenish the B-cell reservoir, but are more likely to be efficiently removed by the now well-established memory-T-cell response. HPV (Human Papilloma Virus): These viruses cause lower genital tract cancers; most commonly cervical cancer. Since HPVs replicate in stratified epithelial cells only, they are the aetiological agents of squamous cell carcinomas (SCCs) at various sites. Some of the high risk HPVs in cancer are HPV16, 18, 31 & 33. Genome: HPV has a small circular genome of ~ 8 kbp and the mRNA is transcribed off one strand of the DNA by host cell RNA polymerase II. There are five early genes coding for proteins, which are involved in DNA replication (E1 and E2) or activation of cell growth (E6, E7 and E5), and three late genes, two of which code for the major and minor capsid proteins (L1 and L2, respectively) and one of unknown function (E4). Like most of the tumour viruses, the cell/growth-activating proteins affect either the signal transduction pathways or cell cycle control. Replication: During the malignant phase of the disease, when the lesion is confined to the epithelium, the viral DNA is in an episomal form, and amplification of the genome and viral particle production occurs in the upper parts of the epithelium. In malignant cells, the genome is integrated randomly in over 70% of cases, while in the rest there are episomal copies. All malignant cells continue to express E6 and E7 proteins, suggesting that these proteins are essential for maintenance of the malignant phenotype. Cancer transformation: The transforming activity of HPV16 is associated with mainly E6 and E7 proteins and some from E5. E6 and E7 are multifunctional proteins that can increase cell proliferation and survival by several pathways. E.g. E6 binds to the E6-associated protein (E6-AP) and p53 (tumour suppressor) in a heterotrimeric complex. The result of this binding is the premature degradation of p53 through the ubiquitin pathway. Since one of the functions of p53 is to control the passage of cells through the G1 phase of the cell cycle, any abrogation of this activity could lead to uncontrolled cell cycle progression. Reading: Chapter 13: Mol & Cell Biol of Cancer by Knowles and Selby Optional Reading 1) Oncogenic viruses by Dennis J McCance www.els.net 2) Epstein-Barr virus: 40 years on Nature Rev Cancer 4 (10)757-68 Oct 2004 Young LS, Rickinson AB







