Synthesis and Characterisation of Advanced ZnS Nanocrystals

Synthesis and Characterisation of Advanced ZnS Nanocrystals
Mandeep Kaur 1 , Ashish Chauhan 2 , Kanchan L. Singh 1 , Praveen Kumar 1
1 DAVIET Jalandhar, Punjab, India E-mail: mandeepbaidwa@yahoo.co.in
2
SMPIC, Mohali, Punjab, India E-mail: aashishchauhan26@gmail.com
ABSTRACT
Zinc sulphide is an important semiconductor that belongs to II-VI group of semiconductors family having a wide band gap (~3.72eV) with large excitonic binding energy (~40 meV) for cubic zinc blende phase. ZnS nanocrystals are promising materials for wide range of applications in electronics as light emitting diodes, electroluminescent devices, photovoltaic devices, lasers, single electron transistors, as well as biological labeling and diagnostics due to its photoluminescence, tuneable bandgap. The hydrothermal method is used for preparation of ZnS nanocrystals. It is a simple and cost effective method to synthesize the highly crystalline semiconducting nanoparticles. In the present work, we have prepared passivated ZnS nanoparticles from zinc nitrate hexahydrate as zinc source, thioacetamide as sulphur source with different molar ratio (0.1 M, 0.05 M) along with different capping agent such as PVP, CPyC, CTAB, PEG, and PVA. The nanocrystals were characterized by advanced techniques.
Keywords: Zinc sulphide, hydrothermal method, capping agent, X-ray diffraction, SEM
INTRODUCTION
Zinc sulphide is an important material belong to II-VI group of semiconductor family having wide bandgap of (~3.72 eV) for cubic Zinc blende phase and (~3.77 eV) for hexagonal phase with large exciton binding energy of (~40 meV) [1] and small bohr radii (2.5 nm) [2]. For semiconductor nanoparticles, the surface is considered to be highly defective which includes size and surface effects. To design a material it requires optical, magnetic, elastic, chemical properties by controlling the surface effect. By eliminate the energy levels inside the gap is known as ‘passivation’, which consists of bonding surface atoms to different materials with a much larger band gap [3] .
The physical and chemical properties of nanocrystals were strongly dependent on the particle sizes due to the quantum confinement effects [4]. It has potential application in optoelectronics devices such as electroluminescent devices, solar panels, reflective coatings, blue light emitting diode, photovoltaic cells etc. As per reported earlier the change in reaction parameters such as temperature, conc. of dopant/capping agents, variation in ratio of starting material have found strong influence on their physical characteristics. It is possible to synthesize a variety of nanocrystallites with variety of sizes as per requirement [5]. The capping agent added during synthesis to prevent the agglomeration of particles and surface passivation. The size of the particle decreases with increase in concentration of capping agent. The choice of a capping agent depends on the material of the nanoparticles core, particle size and the solvent during synthesis. Capping agents with strong binding molecule form dense layer on the particle surface that stabilizes nanoparticles better, while weak binding molecule results to fast particle growth leading to large nanoparticles size
and aggregation[5-6]. The solution based methods are convenient and economical process which can be used to produce such as nanostructured oxides without using metal catalysts or templates with better crystal quality the nanostructured oxides without using metal catalysts or templates with better crystal quality, preferably at lower growth temperature [4]. Different methods for the preparation of nanoparticles are hydrothermal method, sol gel method, microemulsion methods, co- precipitation methods etc [7]. Among other synthesis methods, hydrothermal method is the simple, most economical and cost effective method to fabricate nanocrystals it requires a relatively low temperature, and whole process occur, in a closed which can effectively avoid oxidation phenomena and environmental pollution. The idea behind using this method is the ability to create crystalline phases which are not stable at the melting point and create materials which have a high vapor pressure near their melting points
[8]. In this paper we have prepared ZnS nanoparticles with different capping agent with different molar ratio and also we are going to discuss about the characterization of samples with different characterization techniques such as FTIR, UV-Vis spectroscopy, X-ray
Diffraction, SEM.
EXPERIMENTAL DETAILS
The ZnS nanoparticles were synthesis by using reagents are zinc nitrate hexahydrate (GR)
(loba Chemie, 98%) as Zinc source, thioacetamide (CDH, 99.0%) as sulphur source and
Polyethylene Glycol (Loba Chemie), polyvinyl alcohol (Loba Chemie), Cetyl Trimethyl
Ammonium Bromide (Loba Chemie, 98%) as different capping agent.
The hydrothermal method is used for the synthesis and preparation of samples. In the typical process 2.0 gm in 60 ml capping was added in deionized water and stirred the solution for few minute using magnetic stirrer until it dissolve completely. To this solution, 0.1M zinc nitrate hexahydrate as zinc source and 0.1M thioacetamide as sulphur source were added to solution to yield the transparent solution. This solution was further left on the magnetic stirring for 30 min for homogeneous mixing of the precursors at room temperature without forming precipitates. After this, the precursor solution was taken in an air tight pyrex glass bottle and placed in hot air oven maintained at 100
0
C for 3 h for the completion of the hydrothermal process. End products solution containing white colour were then centrifuged
(Labspin TC 450D) and repeatedly washed 3-4 times with de-ionized water and ethanol for removing the impurities. Finally, the product was dried at 80
0
C in hot air oven for 2 h and it was further used for the different characterizations. The same procedure was followed for the synthesis of all the samples by taking different capping agents by taking different concentrations of the precursors for 0.05 M molarity. The overall process is depicted in figure
1. Further, this powder was used for the characterization using XRD, AFM, FTIR and UV-
Vis spectroscopic techniques. The x-ray diffraction study of the powder samples was carried using x-ray diffractometer (X’Pert Pro, Panalytical Instruments) and the microscopic analysis of the samples was performed using the scanning electron microscope (JSM-6610, Jeol). The
IR absorption spectra were performed on the dried samples using the KBr pallet method in
400- 4000 cm -1 at room temperature by using the FTIR spectrophotometer (RZX, Perkin
Elmer, USA). The optical absorption studies were performed using the UV-Vis spectrophotometer (Evolution 300, Thermo Scientific, USA). The hydrothermally prepared samples using different capping agent were marked as ZnS:1-ZnS:5 for 0.1 M solution and sample ZnS:6 to ZnS:9 were marked for 0.05 M solution .
FIGURE 1: Flowchart of the hydrothermal process used for the synthesis of different samples with 0.10 M and 0.05 M of solutions.
RESULTS AND DISCUSSION
X-ray diffraction study
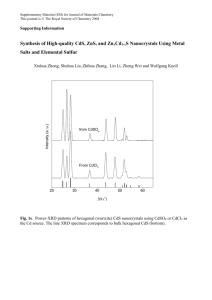
X- ray diffraction is an efficient technique Figure shows the XRD diffraction patterns for pure and capped ZnS nanocrystals. The crystalline size of the nanocrystals can be calculated by using Debye-scherrer relation.
L =
Where λ is the X-ray wavelength (1.54056Å), β is the full width half maximum value of diffraction peak, θ is the degree of the diffraction peak corresponding to the plane.
ZnS powder shows reflection at 28.6
0
, 47.6
0
, and 56.4
0 for the (111), (220) and (311) planes indicate the formation of cubic zinc blende structure for 0.1 M molarity concentration of the samples. The broad peaks indicates smaller in particle size. The calculated average particle size for 0.1 M conc. was found to be (30 nm to 49 nm) for the synthesized nanoparticles for the samples. The results of the diffraction peaks were analyzed for the differently capped nanocrystals as shown in figure: 2 and table: 1 reveals the size of the nanocrystals using different capping agent along with its full width half maximum value and its lattice parameters as shown in table.
ZnS-5
A
10 20 30 40
2θ (in degrees)
50 60 70 80
10 20 30 40 50 60
2θ (in degrees)
FIGURE 2: XRD patterns for (A)pure ZnS nanopowders (B) different capping agents such as ZnS-1: (PEG), ZnS-2: (PVP), ZnS-3: (PVA), ZnS-4: (CPC) using the 0.1 M precursor solutions
70
B
ZnS-4
ZnS-3
ZnS-2
ZnS-1
80
The XRD diffraction patterns for pure and capped ZnS nanocrystals using 0.05M solution.
ZnS powder shows reflection at 28.6
0
, 47.6
0
, and 56.4
0 for the (111), (220) and (311) planes indicate the formation of cubic zinc blende structure. The calculated average particle size for the samples was found to be (20 nm to 33 nm) for the synthesized nanoparticles. The XRD diffraction patterns for pure and capped ZnS nanocrystals. ZnS powder shows reflection at 28.8
0
, 48.0
0
, and 56.9
0 for the (111), (220) and (311) planes which indicate the formation of cubic zinc blende structure. The figure: 3 and table: 2 reveals the size of the nanocrystals using different capping agent with its full width half maximum value and its lattice paramters as shown in table.
TABLE 1: The calculated diffraction parameters for ZnS nanoparticles synthesized without and with different capping agents using 0.1M concentration of precursor salts.
ZnS: Type
Higher peak (2
)
ZnS:1:PEG ZnS:2: PVP ZnS:3:PVA ZnS:4:CPyC ZnS:5:WC a (nm)
FWHM
Particle size (nm)
28.5
0.5404
0.2676
30.6
28.6
0.5402
0.2342
32.6
28.6
0.539
0.2509
35
28.6
0.5401
0.1673
49
28.6
0.5404
0.2676
30.6
.
A
10 20 30 40 50
2θ (in degrees)
60
ZnS-7
70 80
FIGURE 3: XRD Diffraction patterns for (A)PEG capped ZnS nanoparticles (B) using different capping agents for 0.05 M of the precursors denoted as ZnS-6 :(Pure), ZnS-
7: (PEG), ZnS-8: (PVA), ZnS-9: (CTAB). 0.05Msolution.
TABLE 2: The calculated diffraction parameters for ZnS nanoparticles synthesized without and with different capping agents using 0.05 M concentration of precursor salts
ZnS: Type ZnS:6: WC ZnS:7: PEG ZnS:8:PVA ZnS:9:CTAB
Higher peak (2
) 28.5
28.6
28.6
28.9
a (nm)
FWHM
Particle size (nm)
0.5404
0.2676
30.6
0.5402
0.2342
20.2
0.539
24.8
0.2509
0.5401
0.1673
32.0
Surface morphology
The surface topographic study of the surface particles is characterized by using scanning electron microscope. The microscopic study of the nanoparticles has been performed to study the surface morphology and shape of the structure of the nanoparticles which shows the formation of particle in homogeneous and cluster form with variation in particle size.
Figure 4 shows the SEM images of the pure and capped ZnS nanoparticles with CPyC,
PVA, and PVP respectively. This shows the formation of homogeneous particles/clusters with spherical shape having variation in particle sizes except for the PVP capped ZnS nanoparticles where monodisperse spherical nanoparticles has been observed. The formation of spherical monodisperse nanoparticles have also been recently reported for the PVP capped ZnS nanoparticles [9]. They have represented a SEM image of uniform nanospheres of diameter 80 nm for PVP capped ZnS nanospheres. The sphere was composed of primary ZnS nanoparticles which oriented aggregation- based self assembled into the ZnS nanospheres [10].
B
FTIR spectroscopy study
The FTIR spectrum for pure and capped ZnS nanoparticles for 0.1 M concentration and 0.05
M concentration of the precursor. It has been observed that the characteristic absorption bands for the functional groups of the capping agents confirm the passivation of the nanoparticles and various spectra peaks are confirmed with reference paper given in table: 3 as discussed below:
TABLE 3: The FTIR absorption bands for different functional groups of passivated ZnS.
Type
ZnS/PVA
ZnS/CTAB
ZnS/CPyC
ZnS/PVP
ZnS/PEG
Pure ZnS
Frequency cm -1
1407.12,
1106.21
1657.21
2893.32
3333.10
2910,2851
1552
2347
1629
3338
2921
2348
1353
1278,1438
3420
2915
1463
2341
1618, 1543
1543
315,615
3342.4,1633.6
Functional groups
C-H stretching and bending bonds
Bending vibration mode of water molecule
-CH
2
-symmetric stretch bond
-OH stretching of the hydroxyl group
-CH
2
stretching bond
C-H stretching bending vibration mode of water
CO
2
C=C stretching
Lewis bonded pyridinium bond
N-H stretching
Sp
3
(-CH
2
)
CO
2 bond
C=C (acromatic)
N-H-O stretching of pyrrolidone carbonyl
O-H bond
C-H (SP
3
)
C-H (Sp
2
)
O-H carboxylic peak
C=C
N-H bending
ZnS peaks
O-H stretching
&bending peak of water
References
Alexender et al.,2012
[11], Sharma et al.,
2011 [12]
Yesu et al.,2012[13],
Mehta et al.,2009[14]
Mehta et al.,2009[14]
Ghosh et al., 2005[15],
Arabi et al. (2011)
[16]
Murugadoss et.al.
(2011) [17], Selim et al(2011) [18].
John et al.,2010[19], yaun- yaun et al.,2010[20]
The FTIR spectra analysis of the pure and capped ZnS nanoparticles using 0.1 M conc solution are given below. The FTIR spectra analysis of the samples using 0.1 M conc. solution. The FTIR spectra for sample CPyC capped ZnS nanopowders. The absorption spectra shows the major peaks appeared at various point are (-CH
2
) appear at 2921 cm
-1
,
CO
2
appear at 2372 cm
-1
, C=C stretching in lewis-bonded pyridinium bond appeared at
1616 cm
-1
, N-H stretching peaks at 3338 cm
-1
, C=C (acromatic) appear at 1542 cm
-1
.
The FTIR spectra for sample ZnS-5 as shown in (Figure:5 A) of pure ZnS nanopowder.
The absorption peak of pure ZnS appeared at 1906cm
-1
, 1542cm
-1
,1461cm
-1
,1406cm
-
1 with O-H bending( at 1618cm -1 ) of water. The absorption band was confirmed with paper. The FTIR spectra for sample ZnS-1 as shown in (Figure 5 A). Major peaks of PEG capped ZnS appeared at C-H (sp
2
) at 1463cm
-1
, O-H carboxylic peak at 2341 cm
-1
,C=C appear at1618 cm -1 and 1543 cm -1 , N-H bending peaks appear at 1543 cm -1
.
The FTIR spectra for sample ZnS-3 as shown in (Figure : 5 A).Major peaks of PVA capped ZnS spectra shows O-H stretching peaks appear at 2369 cm
-1
peaks, C=C (CH
2
) at 1621 cm
-1
,
1543 cm
-1 due to stretching and bending vibration and C-H (Sp
2
) at 1462 cm
-1
has been observed for the PVA capped ZnS nanoparticles. The FTIR spectra for sample ZnS-2 as shown in (Figure:5 A). Major peaks of PVP capped ZnS appeared at N-H at 3403 cm
-1
,
C-H (SP
3
) at 2925 cm
-1
,C-H (Sp
2
) at 1462cm
-1
, O-H carboxylic peak at 2341 cm
-1
,C=C appear at1626 cm -1 and 1543 cm -1 , N-H bending peaks appear at 1543 cm -1 .
The FTIR spectra analysis of the samples using 0.05 M conc solution The FTIR spectra for sample ZnS-9 as shown in (figure: 5 B) of major peaks of CTAB capped ZnS appeared at 2918 cm
-1 peak appears due to CH
2
stretching bonds and 1112 cm
-1 peaks appeared due to C-H stretching bonds, 1607 cm
-1
appeared due to bending vibrational mode of water molecules. The FTIR spectra for sample ZnS-6 as shown in (figure:5 B) of peaks of pure ZnS appeared at 618 cm -1 , 1109 cm -1 , 3737 cm -1 , O-H stretching peak at
3116 cm
-1
and O-H bending peak at 1613 cm
-1
of water molecule.
The FTIR spectra for sample ZnS-8 as shown in (figure: 5 B) of PVA Capped ZnS spectra depicts peaks of O-H stretching peaks appear at 1623 cm -1 appeared due to bending vibration mode of water molecules and 3398 cm
-1 indicates –OH stretching of the hydroxyl group and 1114 cm
-1 peaks appeared due to C-H stretching bonds. The FTIR spectra for sample ZnS-7 as shown in (figure: 5 B) of major peaks of PEG capped ZnS appeared at C-H (Sp
2
) at 1463 cm
-1
, O-H carboxylic peak at 2341 cm
-1
, C=C appear at
1621 cm
-1
, N-H bending peaks appear at 1543 cm
-1 and peaks appeared at 1112 cm
-1
due to C-H stretching bonds and O-H stretching peaks at 3393cm
-1
[11-21].
Optical properties
The UV-Vis spectra for the capped and uncapped ZnS nanoparticles. As shown in figure
given below .The absorption coefficient has been determined using Beer-Lambert relation,
,
where ‘d’ is the path length, T is the transmittances, ‘ ’ is the absorption coefficients.
Optical bandgap of nanocrystals is determined by using formula formula,
( 1/m g),
C is the constant Eg is the bandgap of the nanoparticles and m depend on type of transition near the fundamental absorption edge. The variation of ( h )
2
versus photon energy h for the capped ZnS nanoparticles shows the optical absorption spectrum for the PVA capped
ZnS nanoparticles (ZnS-3) with 0.1M precursor solution. The strong absorption in the wavelength range 263-328 nm along with the excitonic peak at 263 nm has been observed.
The variation of ( h )
2
versus photon energy (h for the PVA capped ZnS nanoparticles with 0.1M solution is shown in the figure 6. From the extrapolation near the fundamental absorption edge, the optical gap was calculated and found to be approximately 4.86 eV.
Similar results have been reported for the ZnS nanoparticles by Liu et al. [21]. It has been observed that the value of the optical gap is blue shifted from its bulk value of 3.77 eV and reveals the effect of the quantum confinement in the synthesized surface passivated ZnS nanocrystals. Panda et al. have also reported the blue shift in the optical absorption edge for the nanocrystals with the onset at 340 nm and band gap of 4.42 eV [9].
The figure 7 shows the UV-Vis absorption spectra for the pure and capped ZnS nanoparticles with 0.05M solution. This figure shows the strong absorption occurs near the wavelength range 262-375 nm with the excitonic peak appeared at 270 nm, 265 nm, 269nm and 267 nm for the pure ZnS, PEG- ZnS, PVA-ZnS and CTAB-ZnS nanocrystals respectively has been observed. Both the spectra peaks indicate a blue shift of the absorption edge for the ZnS nanocrystals as compared to the bulk optical gap value.
CONCLUSIONS
The ZnS nanocrystals have been synthesized successfully with hydrothermal method using different capping agents such as PVP, PVA, PEG, CTAB and CPyC using the zinc nitrate hexahydrate and thioacetamide as zinc and sulphur sources respectively by taking 0.05M and
0.10 M precursor solutions. The synthesis of ZnS nanocrystals using different capping agents have been characterized using various techniques such as X-ray diffraction, scanning electron microscopy, AFM, FTIR, UV-Vis spectroscopic techniques. The XRD patterns confirm the formation of cubic zinc blende phase and the average particle size varies from 20-49 nm by using the Schererr’s formula for the synthesized. It has been observed that the increase in the precursor concentration results in the increase in the particle size. The use of different capping agents has resulted in the anomalous change in the particle size for the hydrothermally synthesized ZnS nanocrystals. The SEM micrographs show the formation of mono dispersed spherical nanoparticles for the PVP capped ZnS while other samples show some variation in their sizes. The absorption bands for the characteristic functional groups
FTIR absorption spectrum reveal the presence or attachment of the capping agents with the surface of the ZnS nanoparticles. The UV-Vis absorption spectra provide the information about the optical transition, optical gap and excitonic absorption respectively for the differently capped ZnS nanocrystals. The strong absorption in 262-375 nm wavelength range along with the blue shift in the absorption edge for the nanoparticles as compared to that for the bulk semiconductor have been observed for the synthesized nanoparticles. The excitonic peak value for different surfactant/capping agents appeared at 270 nm for pure ZnS, at 265 nm for PEG- ZnS, at 269 nm. The increase in the molarity of the precursors for the PVA capped ZnS nanocrystals results in the shift in the excitonic edge towards longer wavelength.
These advanced nanocrystals can have numerous applications in agriculture and bio-medical applications.
FIGURE 4 : SEM micrographs for the ZnS nanoparticles capped with different capping agents are A: CPyC, B: PURE, C: PVA, D: PVP.
Figure: 5A
Figure 5B
FIGURE 5:- FTIR transmission spectrum (A) for 0.1 M solution (B) for 0.05 M solution capped and uncapped samples
A B
FIGURE 6 :- A: Optical absorption spectra for the PVA capped ZnS nanocrystals.
B: Plot of (
h
) 2
versus photon energy h
for the PVA capped ZnS nanocrystals.
A
B
Pure-ZnS
PEG-ZnS
PVA-ZnS
200 350 500 650 800 l
(nm)
FIGURE 7: The optical absorption spectra for (A ) the pure, PEG and PVA capped ZnS nanocrystals and (B) the CTAB capped ZnS nanocrystals.
REFERENCES
1.Kasherwal, K.K., Ojha, V. N. and Singh. K (2012) Development of memory devices using semiconductor ZnS Nanoparticles, International Journal of Research and
Practices in Engineering Sciences, 1(1) , 67-71.
2.Li, Yuan., Li, Q., Wu, H., Zhang, J., Lin, H., Nie, M. and Zhang, Yu. (2013) High yield growth of uniform ZnS nanospheres with strong photoluminescence properties,
Materials Science and Engineering B, 178, 135– 141.
3.
Mu. j., gu, d & xu z. (2004), Synthesis and stabilization of ZnS nanoparticles embedded in silica nanospheres, Applied Physics A –Materials Science &
Processing.
4.Phuruangrata, A., Thongtemb, T., Thongtemc, S (2011), “Characterization of copper sulfide hexananoplates, and nanoparticles synthesized by a sonochemical method
,Chalcogenide Letters, 8( 4), 291 – 295.
5.Wageh, S., Zhao, S. L.and Rong, X (2003), “Growth and optical properties of colloidal
ZnS nanoparticles”, Journal of Crystal Growth ,255, 332–337.
6.Alexander N. A., Echi Idugba, M. & Onyekachi1, K. (2012), “Influence of polyvinyl alcohol and alpha-methacrylic acid as capping agents on particle size of ZnS nanoparticles”,Applied Physics Research, 4(4), 26-34.
7.Kumar, S., Verma, N.K., Singla, M.L. (2011), “ Reflective characteristics of Ni doped
ZnS nanoparticle-pigment and their coatings” , Chalcogenide Letters, 8(9), 561-569.
8.Hoa, T. T. Q., Vu, L. V., Canh, T. D. And Long, N. N. (2009). “Preparation of ZnS nanoparticles by hydrothermal method.”, Journal of Physics: Conference Series 187,
012081.
9.Panda, S., Datta, A., Chaudhuri, S., (2007), “ Nearly monodispersed ZnS nanospheres:
Synthesis and optical properties”, Chemical Physics Letters, 440, 235–238.
10.
Li, Yuan., Li, Q., Wu, H., Zhang, J., Lin, H., Nie, M. and Zhang, Yu. (2013)
“High yield growth of uniform ZnS nanospheres with strong photoluminescence properties”, Materials Science and Engineering B, 178, 135– 141.
11.
Alexander N. A., Echi Idugba, M. & Onyekachi1, K. (2012), “Influence of polyvinyl alcohol and alpha-methacrylic acid as capping agents on particle size of
ZnS nanoparticles”,Applied Physics Research, 4(4), 26-34.
12.
Sharma, R., (2011) Structural and optical characterization of ZnS nanoparticles.
International Multidisciplinary Research Journal, 1(9), 08-11.
13.
Yesu Thangam Y., Anitha R. & Kavitha B. (2012), “Novel method to synthesize and characterize Zinc Sulfide nanoparticles”, International Journal of Applied
Sciences and Engineering Research, 1(2), 282-286.
14.
Mehta, S. K., Kumar, S., Chaudhary, S. & Bhasin, K. K. (2009), “Effect of cationic surfactant head groups on synthesis, growth and agglomeration behavior of ZnS nanoparticles”, Nanoscale Research Letters, 4, 1197–1208.
15.
Ghosh, G., Naskar, M. K., Patra, A. And Chatterjee, M. (2006), “Synthesis and characterization of PVP-encapsulated ZnS nanoparticles”, Optical Materials, 28,
1047–1053
16.
Arabi, A. M., Ebadzadeh, T., Yousefi, Ali. A., Pishvaei, Maliheh., Marzban Rad,
E. and Najafi, F. (2011) “The function of nano-polystyrene template and comb polycarboxylic acid surfactant in synthesis of ZnS nanoparticles via Hydrothermal method”, Iranian Polymer Journal, 20 ( 7 ), 559-569.
17.
Murugadoss, G. and Ramasamy, V. (2013), “Synthesis, effect of capping agents and opticalproperties of manganese-doped zinc sulphide nanoparticles”, Luminescence:
The journal of biological and chemical Luminescence, 28: 69–75.
18.
Selim, KM. K., Xing, Z. C., Choi, M. J .,Chang, Y., Guo, H. and Kang, I.K.
(2011),“Reduced cytotoxicity of insulin-immobilized CdS quantum dots using PEG as a spacer”, Nanoscale Research Letters, 6(528), 1-9.
19.
John, R., Florence, S. S. (2010), “ Optical, structural and morphological studies of bean- like ZnS nanostructures by aqueous chemical method”, Chalcogenide Letters,
7(4), 269 – 273.
20.
Yuan-Yuan, S., Juan, Y., Ke-Qiang, Q. (2010) “Synthesis of ZnS nanoparticles by solid-liquid chemical reaction with ZnO and Na
2
S under ultrasonic”,Transactions of
Nonferrous Metals : Society of China, 20, S211-S215
21.
Liu, W. And Chen, S. (2000), “An investigation of the tribological behaviour of surface-modified ZnS nanoparticles in liquid paraffin’, Wear, 238, 120–124.