AP Lab - Freezing Point Depression
advertisement

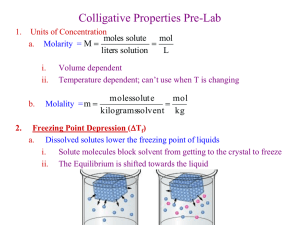
Name ________________________________________________________________Date_______________________ NORTH ALLEGHENY SENIOR HIGH SCHOOL AP Chemistry MOLAR MASS BY FREEZING POINT DEPRESSION Introduction/Theory A procedure that allows the determination of the molar mass of a substance is very useful to chemists. The molar mass is an important value that must be known in order identify an unknown substance or to characterize a newly prepared compound. There are a number of ways of determining the molar mass of a substance. One of the simplest involves finding the change in the freezing point of a solvent when an unknown substance is dissolved in it. It has been found that the change in freezing point is directly proportional to the molaliry of the solution. This change in freezing point is one of several "colligative" properties of solutions - properties that depend only on the number of dissolve particles in solution, and not on the type of particle. Other colligative properties include change in boiling point, vapor pressure and osmotic pressure. Measurements of these properties can also be used to find molar mass of solutes. The molality of a solution, m, is defined as moles of solute divided by kilograms of solvent: m = moles solute kg solvent Since moles of solute is the same as grams solute divided by molar mass of solute, Mw, then: m = g solute (kg solvent)(Mw solute) The relation to change in freezing point is: Tfp = kfpm where Tfp is the change in freezing point, kfp is the freezing point depression constant for the solvent, and m is the molality of the solution. The value of kfp must be determined for each solvent. The equations are combined as follows: Mw solute = (kfp)(g solute) (kg solvent)(Tfp) The solvent that will be used in this experiment is concentrated acetic acid, often referred to as glacial acetic acid due to its low freezing point and its resemblance to a 'glacier' when frozen. Acetic acid has the formula HC 2H3O2. The freezing point of glacial acetic acid is around 16 oC. Figure 1 below shows a cooling curve for a pure solvent and for a solution. Notice that supercooling may occur in both the solvent and solution. If it does, as the crystals begin to form the temperature will rise slightly and then remain constant as the pure solvent freezes, or will slowly fall as the solution freezes. The best method of determining a freezing point of a solvent or solution is to monitor the temperature change with time (as in figure 1). By intersecting the cooling lines where the supercooling does not occur, we can interpolate the freezing point of the system as is done in the figure. Even though the melting point of acetic acid is known, it is necessary to determine it with the thermometer that will be used in the experiment. Be sure to use the same thermometer in each part of the experiment, for it is the change in temperatures that we are concerned with, not necessarily the accuracy of the readings. Procedure 1. Obtain a flat bottom test tube. Obtain the tube’s mass. 2. Remove the tube, add about 10 mL of glacial acetic acid (solvent) to the tube, place it back on the balance and record the new mass to the nearest .001 gram. (CAUTION: Glacial Acetic Acid can cause burns. Use extreme care when handling.) 3. Prepare an ice bath in a 400 mL beaker. 4. Prepare a warm water bath by placing warm tap water in a 400 or 600 mL beaker. 5. Set up the experiment as discussed in class. Clamp the tube to a ring stand and use the thermometer to stir. Use caution when stirring. 6. Place the test tube in the ice bath and take temperature readings every 10 seconds. Stir the acid with the thermometer and record the temperature when the acid freezes. Continue recording data for at least one minute even after freezing. Keep in mind the method we will use to determine the true freezing point of the system (see figure 1). Data is needed beyond the freezing point to do this (possibly). 7. Warm the test tube in a beaker of warm tap water until all of the crystals melt. 8. Discard the acetic acid. 9. Clean and dry your tube. 10. Obtain another 10 mL of acetic acid (record its mass). 11. Add about 1.5 mL of 2-propanol (solute) to the tube (use the plastic graduated pipet) and record the new mass. Record the mass of the 2-propanol to the nearest .001 gram. 12. Stir the system well, place the tube in the ice bath (add more ice before beginning), and record the new freezing point (follow the same idea as above, taking data every 10 seconds). 13. Heat the test tube until all of the crystals melt and discard the solution. 14. Repeat steps 10-14, this time using about 1.5 mL of the unknown compound to the test tube and determine the mass of the unknown. 15. Determine the freezing point of the acetic acid/unknown system, following the same steps as were done above. Take data points every 10 seconds and take data beyond the freezing point. 16. Heat the system and discard solution. 17. Clean up. Calculations Using the theory discussed in class and presented in the introduction, do the following: 1. Plot the freezing point data using a computer graphing package (such as Excel) and analyze the graphs as is shown in figure 1 (Mark the apparent freezing point temperatures). You should have 3 graphs – pure solvent, 2-proponal, and unknown. 2. Obtain the freezing points from the graphs and use that data to complete the rest of the calculations. 3. Determine the kfp for acetic acid. 4. Determine the molar mass of the unknown solute, using the kfp from (3) and the data from the unknown trial. 5. If it is known that the unknown is cyclohexanol, determine the percent error in molar mass relative to your experimental value. Conclusion/Discussion Provide answers to the following questions at the end of your report. 1. 2. 3. 4. 5. What is a colligative property? Draw a phase diagram of a pure substance, and show how addition of a solute affects this diagram. What is the least accurate measurement that occurs in this lab? How does this limit your significant digits? Why is it advantageous to choose a solvent that has a large value for k fp? Explain why the pure solvent shows a level horizontal curve as solidification occurs, but the curve for the solution slopes downward slightly. 6. The following errors occurred when the above experiment was carried out. How would each affect the calculated molecular mass of the solute? (too high, too low, no effect)? Explain your answer. a) The thermometer was calibrated to read 1.4 °C too high. b) Some of the solvent was spilled before the solute was added during the unknown trial.