Developmental Lesson: Model of the Atom
advertisement

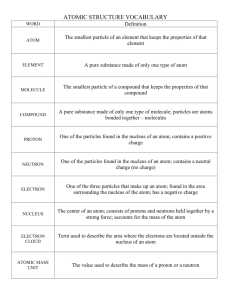
Developmental Lesson: Model of the Atom Teacher Notes Goal Facets 01 The student knows most of the mass of the atom is in the nucleus (protons and neutrons). Goal Facet 02 The student knows that the electrons of an atom are outside the nucleus, and that the space in which they move defines the volume of an atom which is mostly empty. I. II. III. Overview: Models are a simple way of thinking of something that is complicated or difficult to observe directly. Models are limited so we must be careful not to draw conclusions beyond the reach of the model. For instance, if we are using a billard ball model of the atom, it may help us to show motion but the color and size are not correct. A good model will allow us to solve the problem at hand AND will suggest other solutions to problem we had not expected to solve. Today your students will attempt to model the atom with respect to the nucleus relative to the entire atom. In this activity students are going to conduct a simplified Rutherford like experiment. They are going to launch uncharged particles (balls) at would be atoms inside of a detector. Students are asked to collect the information about what happens to the uncharged particles when it goes through the atoms and hits the detector. Students will start off working alone and then will work in groups. You will place the student in groups depending on the model of the atom that they drew in the elicitation question. Check elicitation responses for some common answers. Once the students are in groups, then they will set up their atoms and fire the particles (roll the ball) and collect information about the detector. When you collect the materials for this lab you have to think about what would be a good proton or neutron in comparison to the particles that you will launch. Juice cans for protons and neutrons, ping pong balls for particles and little pieces of yarn for electrons work best. But you can improvise. You can think about this a bowling but not with the intent of knocking down pins, but rather seeing where the balls are deflected to by different configurations of nucluei (i.e., plum pudding model vs. bohr model). Materials: Juice cans, balls, rice/confetti/yarn/string/playdoh for electrons Procedure A. Have students look at the picture of the atom that they drew in the Elicitation Question. Have them attend to the whole volume of the atom and location of the nucleus? Look at some of the common responses under elicitation questions. Collect from students some common representations of the model of the atom that they drew. Sketch them on the board. Developmental Lesson: Model of the Atom Teacher Notes Figure 1: Protons and Neutrons and nucleus Figure 2: Missing protons and neutrons B. Ask students if their drawings are to scale. How would you change it thinking of a scale model? A common example is to think that if the nucleus of an atom where a basketball, the space around the atoms where the electrons would be encompasses fifteen miles. C. Tell the students that they are going to experiment to determine which model of the atom best explains the data we collect by rolling a particle at the atom and using a screen to detect where the particle goes after we roll it. D. Using the first data chart provided, draw in your model of the atom from your picture. Where do you predict the particle will go if it is rolled at your model? Will it bounce off, Will it go past? Will it come back? E. Place the students into groups based on their model types and have them build their model. Teacher should organize students in groups so that students with similar models are in the same group. It is best to identify the different groups as you collect the models in A above. F. Once in groups, tell students to build your model using the materials provided. G. Ask, How big does the detector screen need to be? H. Without looking, roll a particle (about the size of one of the nuclear particles) at it, and record where the particle went in the second data chart provided. I. You will need some partners to keep track and to record where the particle went while you roll. J. What happens if you add a second atom? Have students try out another model of an atom. K. Once the students have had a chance to experiment and collect data, have a few groups present their results and discuss the results with the class. L. You will need to run some experiments with your materials to get a distribution of hits on the detector. Note that you should see some sort of shadow from the model Developmental Lesson: Model of the Atom Teacher Notes you used in class. pHet Rutherford scattering is one example to show students because it compares the plum pudding model with a massive nuclear center. Note that the particles are charged in the simulation. Here are some responses from using ping pong balls and juice cans. IV. Have students respond to these questions A. Why did some particles bounce back while other particles passed straight through the atom? Some of the particles bounced back because they hit the juice cans. This represents that the particles hit a massive nuclear particle. B. What does this suggest about the distribution of protons and neutrons within the atom? The distribution suggests that the nuclear material is found in one central location in the atom and that an atom is composed of mostly empty space. The distribution rules out the nuclear material is spread out in the atom because we see a shadow in the data. C. If the protons and neutrons are clustered together in the nucleus, where are the electrons? What does this suggest about the distribution of mass within the atom? The electrons are found in mostly empty space of the atom and most of the mass of the atom is in the center of the atom. D. If the electrons occupy the largest volume of space of the atom, and most particles go directly through the atom, what does this say about the volume of space occupied by the electrons? This suggests that the volume of space of that electrons occupy is large compared to the nucleus and that it is mostly empty space. E. What does the particle go “through” as it passes through the atom? Note: students might think that nuclear material has some cytoplasm or some thing. The particle passes through empty space. It is hard to imagine that the paper that you are holding is mostly empty space. V. Take-Home Questions/Conclusion Rutherford performed a similar experiment passing a beam of positively charged alpha particles through a thin sheet of gold foil. He also got the same results—most of the particles went straight through the sheet while some were deflected back. Refer students to some simulation of this experiment on the web. Let students know that the simulations on the web show a charged particle and that there is positive-positive repulsion that is not part of the experiment they did in class. Have students respond to these questions. 1. How would today’s experiment with a single atom compare with one in which the beam of particles was positively charged? 2. Given what you’ve learned about atomic structure, explain Rutherford’s results. Use a sketch of atoms making up the gold foil to support your answer.