Estrogen plays an important role in the development
advertisement

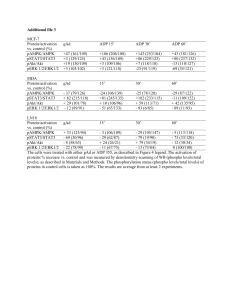
Non-genomic actions of estrogen: physiological implications and signalling pathways Thais F.G. Lucas, Erica R. Siu; Maria F.M. Lazari, Catarina S. Porto - Section of Experimental Endocrinology, Department of Pharmacology, Universidade Federal de São Paulo, São Paulo, Brazil, 04044020. 17-estradiol (E2) plays an important role in the development, differentiation and growth of the male reproductive system by binding to estrogen receptors (ER and ER) (see 1-4 for reviews). Our previous studies showed that the effect of E2 on Sertoli cell (Sc) function involves: translocation of the ER and ER to the plasma membrane mediated by the nonreceptor tyrosine kinase Src, activation of the MAPK (Erk1/2) and cell proliferation (5). In addition, E2 or antiestrogen ICI 182,780 (ICI) can also rapidly activate Erk1/2 after downregulation of both ERs in Sc, through unidentified ER-mediated mechanisms. The rapid action of estrogen can be mediated by membrane translocation of ERs or through proteins other than ERs, such as G-protein coupled receptor (GPR30), as previously shown in different cells (see 6-8 for reviews). In the present study was shown the expression of GPR30 in cultured Sertoli cells from immature rats and the role of E2 and ICI to induce Erk1/2 activation and Sc proliferation. RT-PCR assays detected transcript of the expected size (662 pb) for GPR30. E2 (10-10M, 350C) induced a rapid and transient increase in the phosphorylation state of Erk1/2. Peak Erk1/2 phosphorylation levels occurred at 10 min (7- to 12-fold increase) with Erk1/2 activity returning to basal levels by 15-30 min. Cells treated with cAMP-dependent protein kinase A inhibitor H89 (20 M, 2 h) maintained elevated levels of Erk1/2 activity for an extendent period of time (more than 30 min) after E2 stimulation. Erk1/2 activation in Sc could also be achieved using ICI (10-9M). An increase in cell proliferation was observed after these treatments. The activation of Erk1/2 and cell proliferation induced by either E2 or ICI were blocked by pre-treatment of Sc with pertussis toxin (PTX, 100 µM, 16 h) or Src family tyrosine kinase inhibitor PP2 (5 nM, 30 min). EGF receptor kinase inhibitor AG 1478 (50 µM, 15 min), metalloprotease inhibitor GM 6001 (200 nM, 30 min) and MEK1/2 inhibitor U0126 (100 M, 30 min) also blocked Erk1/2 activation. Taken together, these data suggest that E2GPR30 signaling occurs through a PTX-sensitive pathway and requires Src-related tyrosine kinase activity. The activation of Erk1/2 induced by E2 or ICI is dependent upon transactivation of the EGF receptor via release of heparin-bound EGF. Furthermore, the activation of cAMP-dependent PKA is required to restore E2-induced Erk1/2 activity to basal levels. Supported by FAPESP, PRONEX/FAPESP, CNPq. (1) Hess et al., 1997 J Androl., 18:602 (2) Carreau et al., 2006, Mol Cell Endocrinol., 26:246 (3) Sierens et al., 2005 Ann N Y Acad Sci., 1061:65 (4) Akingbemi 2005 Reprod. Biol. Endocrinol., 3:51 (5) Lucas et al., 2006 Abstract The Endocrine Society’s 88 th Annual Meeting., pp 718 (6) Filardo, 2002, Mol Endocrinol., 16:70. (7) Filardo and Thomas, 2005, Trends Endocrinol Metab., 16:362. (8) Manavathi and Kumar, 2006, J Cell Physiol., 207: 594.