Weather and Water
advertisement

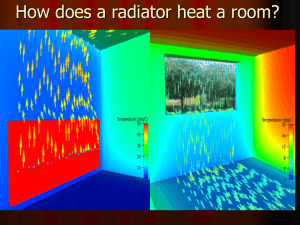
Weather and Water Lesson 5 Review Completed Read these things: Density, Convection and Lesson 5 Notes Definitions: Mass = the amount of matter in an object Volume = the amount of space an object takes up Density = mass per unit volume Also know the units used for: Mass___g or pounds______ Volume___ml or cc_____ Density__g/ml or g/cc______ How can you compare the density of two substances without using an Equation? If two substances have equal volumes then you can weigh them or put them on a scale Be able to calculate density questions on the exam: Example #1 A solution has a mass of 100 g and a volume of 55 ml, what is its density? 100g/55ml = 1.82 g/ml Example #2 A solution has a mass of 150 g and a volume of 200 ml, what is its density? 150g/200ml = .75g/ml How are density and temperature related? Provide examples When an object warms up the molecules separate, making the object less dense. When an object cools down the molecules get closer together, making the object more dense. Examples; layering hot and cold water, hot air balloons Essay: Explain how convection works and affects global weather? (Include These things = role of the sun, heat transfer, and density) 1. Radiant energy from the sun is absorbed by Earth's Surface land and water 2. Energy then transfers to air molecules by conduction or reradiated energy 3. The air then gets warm, less dense and rises into the atmosphere 4. It spreads out and gets cooled, becoming more dense and falls to the Earth's surface 5. The air then is heated again (by conduction and reradiated energy) and the process starts all over again