Extensive vs. Intensive Properties, Chemical & Physical Properties
advertisement

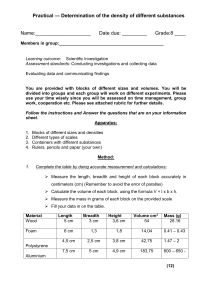
Chemistry Notes: Extensive vs. Intensive Properties, Chemical & Physical Properties, Density Mass is an _________________ property—a property that depends on the ____________________ of stuff you have. Other properties that are extensive would include ____________________, shape, etc. _______________________ properties are ones that only depend on what ______________ of stuff you have, like color, etc. Intensive or Extensive? • Mass __________________________ • Color __________________________ • Density __________________________ • Shape __________________________ • Melting point • Texture __________________________ • Flammable __________________________ __________________________ PHYSICAL PROPERTIES A quality or condition of a substance that can be observed or measured without changing the substance’s ____________________________ What are some examples? STATES OF MATTER Matter’s state is a _______________________ property SIDENOTE: A gaseous substance that is found as a solid or liquid at room temperature is called _____________________. PHYSICAL CHANGES A physical change is one that does not involve __________________ the chemical composition. CHEMICAL REACTIONS The ability of a substance to react and form new substances is called a _________________________ ________________________. In a ______________________ ____________________________, one or more substances change into new substances. A reaction starts with the ____________________________, and the substances formed are called ________________________. 4 CLUES TO CHEMICAL REACTION ___________________, ____________________, ____________________ & _______________ CHEMICAL OR PHYSICAL CHANGE? • Baking cookies __________________________ • Boiling water • Dissolving salt __________________________ • Burning firewood • Milk spoiling __________________________ • Metal rusting __________________________ • Tearing paper __________________________ • Melting ice __________________________ __________________________ __________________________ Density density – the _______________ of an object per unit _____________________ ex) the density of a substance describes how ___________________ packed the molecules are density = D= Important Units Mass is measured in ____. Liquid volume is measured in ___. Solid volume is measured in ____. Liquid density is measured in ________. Solid density is measured in ________. An object will ___________ if it is less dense than the liquid it is immersed in. ex) _________ floats on water because oil is less dense than water An object will ___________ if it is more dense than the liquid it is immersed in. ex) a rock will sink in water because it is ____________ dense than water QUESTION #1: Why does diet coke float in water, but regular coke sinks in water? QUESTION #2: Which is more dense: ice or water?