16. 2 Activation Energy (Arrhenius Equation)
advertisement
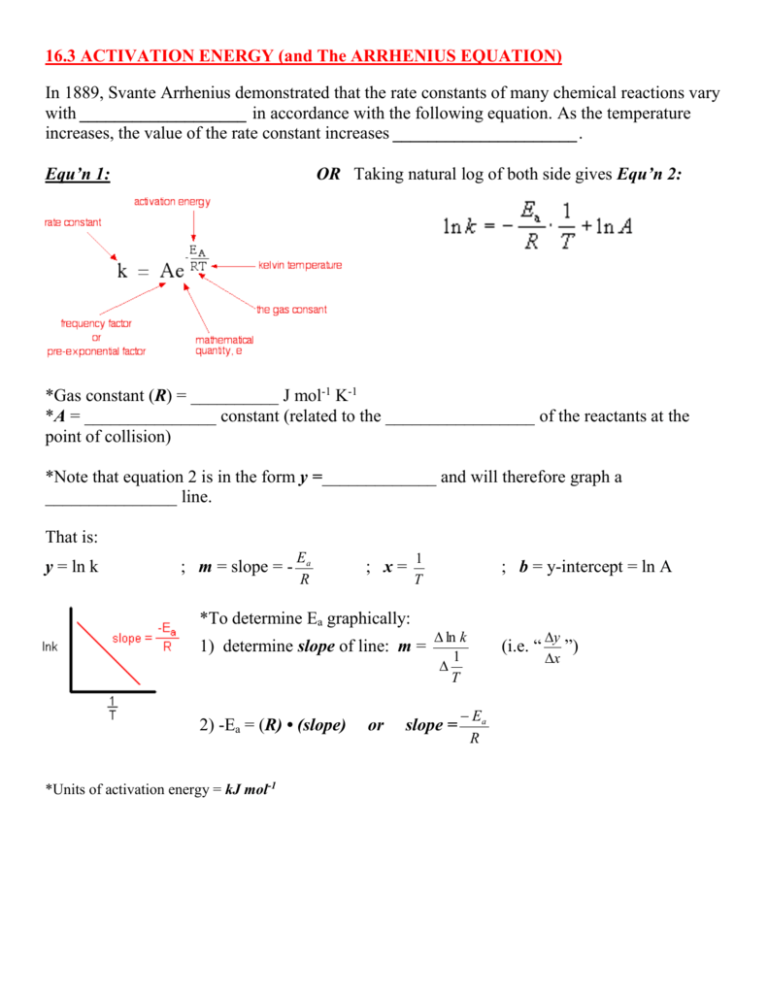
16.3 ACTIVATION ENERGY (and The ARRHENIUS EQUATION) In 1889, Svante Arrhenius demonstrated that the rate constants of many chemical reactions vary with ___________________ in accordance with the following equation. As the temperature increases, the value of the rate constant increases _____________________. Equ’n 1: OR Taking natural log of both side gives Equ’n 2: *Gas constant (R) = __________ J mol-1 K-1 *A = _______________ constant (related to the _________________ of the reactants at the point of collision) *Note that equation 2 is in the form y =_____________ and will therefore graph a _______________ line. That is: y = ln k ; m = slope = - Ea R ; x= 1 T *To determine Ea graphically: 1) determine slope of line: m = 2) -Ea = (R) • (slope) *Units of activation energy = kJ mol-1 or ; b = y-intercept = ln A ln k 1 T slope = Ea R (i.e. “ y ”) x Try the Following Activity: Determining Ea Graphically Problem: The following data were collected for the reaction: 2NO2(g) 2NO(g) + O2(g) Rate Constant (mol dm-3 s-1) 7.8 Temperature (ºC) 400 10 410 14 420 18 430 24 440 Temperature (K) Determine the activation energy for the reaction in kilojoules per mole. Start by completing the following: (*Remember ln k = ____ and 1/T = _____) ln k ln(7.8) = 2.05 1/T (K-1) 1/673 = 1.486 x 10-3 Next: plot points on graph paper provided (use ENTIRE graph!) draw straight line of best fit calculate slope of the line(show slope calculation on graph) calculate the activation energy using: Extension: What is the approximate value of ln A? Answers: *Slope should be around: -1.4 x 104 K-1 *Ea should be around: 1.2 x 102 kJ mol-1