Paper Molecule Activity
advertisement

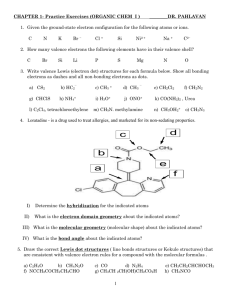
Paper Molecule Activity DO STEPS 1 AND 2 AS A LAB GROUP 1. Use the scissors to cut out all of the atoms off of the pattern sheet. Be sure that you cut along the lines and that the outer “electrons” are left complete for bonding. 2. Use the paper atoms to build the following molecules: a. H20 Water b. CH4 Methane (natural gas) c. CF2Cl2 Difluorodichloro Methane d. NH3 Ammonia e. HCl Hydrochloric Acid (stomach acid) Remember that all atoms seek to have 8 valence electrons (except Helium and Hydrogen which seek to have 2) DO THE FOLLOWING ON YOUR OWN LINED SHEET OF PAPER: 3. Use different colors to represent each type of atom and their valence electrons. Create a “color key” on your paper and draw then draw the 5 atoms on your lined paper. Be sure to label them and show which valence electrons came from which atoms. 4. What are valence electrons and why are they important? 5. How many atoms can attach to the Oxygen atom? Why can’t it bond with more atoms at the same time? 6. Why doesn’t Neon bond with any other atoms? 7. How does the arrangement of the Periodic Table of the elements show us how many valence electrons an atom has? Paper Molecule Activity DO STEPS 1 AND 2 AS A LAB GROUP 1. Use the scissors to cut out all of the atoms off of the pattern sheet. Be sure that you cut along the lines and that the outer “electrons” are left complete for bonding. 2. Use the paper atoms to build the following molecules: a. H20 Water b. CH4 Methane (natural gas) c. CF2Cl2 Difluorodichloro Methane d. NH3 Ammonia e. HCl Hydrochloric Acid (stomach acid) Remember that all atoms seek to have 8 valence electrons (except Helium and Hydrogen which seek to have 2) DO THE FOLLOWING ON YOUR OWN LINED SHEET OF PAPER: 3. Use different colors to represent each type of atom and their valence electrons. Create a “color key” on your paper and draw then draw the 5 atoms on your lined paper. Be sure to label them and show which valence electrons came from which atoms. 4. What are valence electrons and why are they important? 5. How many atoms can attach to the Oxygen atom? Why can’t it bond with more atoms at the same time? 6. Why doesn’t Neon bond with any other atoms? 7. How does the arrangement of the Periodic Table of the elements show us how many valence electrons an atom has?